JACQUES DONNEZ1, SHERMAN SILBER2, CLAUS YDING ANDERSEN3, ISABELLE DEMEESTERE4, PASCAL PIVER5, DROR MEIROW6, ANTONIO PELLICER7 & MARIE-MADELEINE DOLMANS1
1Université Catholique de Louvain, Institut de Recherche Expérimentale et Clinique, Department of Gynecology, Cliniques Universitaires Saint-Luc, 1200 Brussels, Belgium, 2Infertility Center of St Louis at St Luke’s Hospital, St Louis, MO 6301, USA, 3Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, Denmark, 4Research Laboratory on Human Reproduction, Faculty of Medicine, Université Libre de Bruxelles (ULB), Erasme Hospital, 1070 Brussels, Belgium, 5Service de Gynécologie Obstétrique, CHU de Limoges, 87042 Limoges, France, 6IVF Unit, Division of Obstetrics and Gynecology, Sheba Medical Center, Tel Hashomer, Israel, and 7Instituto Valenciano de Infertilidad (IVI), University of Valencia, Spain
Annals of Medicine, 2011
Abstract
INTRODUCTION: Premature ovarian failure (POF) can occur naturally at an early age or be due to iatrogenic agents. Indeed, ovaries are very sensitive to cytotoxic treatment, especially to radiation and alkylating agents. METHODS: Several options are currently available to preserve fertility in cancer patients and allow them to conceive when they have overcome their disease: embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation. Cryopreservation of ovarian tissue is the only option available for pre-pubertal girls and women who cannot delay the start of chemotherapy. FINDINGS: Since the first live birth after autotransplantation of cryopreserved ovarian tissue in humans was reported in 2004, orthotopic reimplantation has led to the birth of 13 healthy babies. Restoration of ovarian activity and prognostic factors are evaluated by comparison with 7 cases of fresh ovarian tissue transplantation.We report 13 live births after orthotopic transplantation of frozen-thawed ovarian tissue in cancer patients (n = 8) and in patients treated with high doses of chemotherapy for benign diseases (n = 2) (microscopic polyangiitis, sickle cell anemia). INTERPRETATION: Based on our review, we believe that ovarian cortex cryopreservation, associated or not with cryopreservation of immature oocytes, should be offered before gonadotoxic chemotherapy in all cases where there is a high risk of POF and where emergency IVF is not possible.
Introduction
Premature ovarian failure (POF) can occur naturally at an early age or be due to iatrogenic agents. Indeed, ovaries are very sensitive to cytotoxic treatment, especially to radiation and alkylating agents, which are classified as high risk for gonadal dysfunction. Cyclophosphamide is the agent most often implicated in causing damage to oocytes and granulosa cells in a dose-dependent manner (1–3). Several options are currently available to preserve fertility in cancer patients and allow them to conceive when they have overcome their disease: embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation (for review see (4–7)). Although freezing of immature (8) and mature (9) oocytes, as well as advancing technological developments in in-vitro follicular growth (10,11), are promising techniques, cryopreservation of ovarian tissue is the only option available for pre-pubertal girls and women who cannot delay the start of chemotherapy (6,12). The main aim of this strategy is to reimplant ovarian cortical tissue once treatment is completed and the patient is disease-free (13–17). There are, in theory, three options for reimplantation (Figure 1). Ovarian cortical pieces can be grafted orthotopically or heterotopically. To avoid transmission of malignant cells, isolated follicles may be cultured in vitro (in-vitro maturation (IVM)) or reimplanted in a tissue-engineering solution (scaffold) (11,18). Finally, cryopreservation of a whole ovary followed by vascular transplantation after thawing could, in theory, avoid follicular loss due to ischemia (13–17). In the wake of encouraging preliminary studies by Gosden et al. (19), demonstrating spontaneous pregnancy and live births after autotransplantation of frozen-thawed cortical tissue in sheep, Donnez et al. (2004) reported the first live birth after autotransplantation of cryopreserved ovarian tissue in humans (20). Orthotopic reimplantation has so far led to the birth of 13 healthy babies in 10 women (21–28). Here we describe the first series of 10 patients to have undergone autotransplantation of their frozen-thawed ovarian tissue, who have given birth to 13 healthy babies to date. These ten patients underwent chemotherapy that resulted in POF but had their ovarian tissue cryopreserved and then reimplanted either once or twice.
Key messages
- Ovaries are very sensitive to cytotoxic treatment, especially to radiation and alkylating agents, which are classified as high risk for gonadal dysfunction. Several options are currently available to preserve fertility in cancer patients and allow them to conceive when they have overcome their disease: embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation.
- Various strategies of fertility preservation are applied depending on the risks and probabilities of gonadal failure, the patient’s general health at diagnosis, and the partner status. These include IVF and embryo cryopreservation, which are standard established procedures with predictable and well documented outcomes in terms of pregnancy and child-birth rates. Harvesting and cryopreservation of ovarian tissue before commencing sterilizing chemotherapy has been increasingly practiced during the past decade. With ovarian cryobanking, abundant primordial follicles, containing small, less differentiated oocytes, are stored.
- Surgical techniques: 1) In the reported series, the peritoneal window created close to the ovarian hilus (JD, ID, PP) and the ovarian medulla both appear to be equally efficient sites of reimplantation, at least for restoration of ovarian activity (as proved by follicular development). Although Demeestere et al. considered follicular activity in the peritoneal site to be lower than that observed on the ovarian medulla, others demonstrated comparable follicular activity in both locations. 2) Large strips (8–10 mm X 5 mm) and small cubes (2 mm X 2 mm) of tissue were both shown to effectively restore ovarian endocrine function.
- In some diseases, like leukemia (see recent paper in Blood, 2010), there is a risk of ovarian metastasis. Ovarian tissue cryopreservation should nevertheless be proposed to these patients, but options other than autotransplantation of ovarian cortical fragments must be considered after thawing, such as in vitro maturation of primordial follicles or grafting isolated of primordial follicles.
Patient Studies
Patient 1 (JD). In 1997, a 25-year-old woman presented with clinical stage IV Hodgkin’s lymphoma. Ovarian tissue cryopreservation was carried out before chemotherapy. By laparoscopy, five biopsies, about 1.2–1.5 cm long and 5 mm wide, were taken from the left ovary and cryopreserved using DMSO as a cryoprotectant, according to Gosden’s protocol (19).
After laparoscopy, the patient received MOPP/ ABV hybrid chemotherapy (mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine) from August 1997 to February 1998, followed by radiotherapy (38 Gy).
She became amenorrheic shortly after initiation of chemotherapy. Levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) following chemo- and radiotherapy were, respectively, 91.1 mIU/mL, 85 mIU/mL, and 17 pg/mL, confirming castration. This ovarian failure profile was confirmed 3 months later.
In 2003, the patient, now wishing to conceive, underwent a first laparoscopy 7 days before reimplantation in order to create a peritoneal window by means of a large incision just beneath the right ovarian hilus, followed by coagulation of the edges of the window. The goal was to induce angiogenesis and neovascularization in this area. A second laparoscopy was carried out 7 days later. A large strip and 35 small cubes of frozen-thawed ovarian tissue were pushed into the furrow created by the peritoneal window very close to the ovarian vessels and fimbria on the right side. A biopsy of 4–5 mm in size was taken from each of the atrophic ovaries, proving the absence of any primordial follicles. A third laparoscopy was performed 4 months later to reimplant the remaining ovarian cortical cubes, upon the request of the patient, now aged 32 years.
From 5 to 9 months after reimplantation, ultrasonography revealed the development of a follicle with each cycle at the site of reimplantation. This corresponded with an E2 level of more than 100 pg/ mL. LH and FSH levels were significantly (P < 0.05) lower than those observed before reimplantation. This led to the restoration of consecutive menstrual bleeding each month.
Eleven months after reimplantation, the patient became pregnant, and the pregnancy resulted in the live birth of a healthy girl, weighing 3.720 kg.This was the first ever live birth after orthotopic transplantation of cryopreserved ovarian tissue (20).
Following delivery and breast-feeding for 5 weeks, the patient had regular menstrual cycles every 4–6 weeks. She experienced 2 months of amenorrhea on three occasions, with FSH values > 40 mIU/mL, subsequently returning to normal cycles.
For up to 4 years after transplantation, the patient had menstrual cycles every 4–6 weeks. Increased FSH levels were sometimes observed before the follicular phase, and the patient showed relatively high basal FSH concentrations (ranging from 8.1 mIU/ mL to 72 mIU/mL, median 35.9 mIU/mL), while E2 was found to be between 21 and 178 pg/mL (median 88 pg/mL). Five years after reimplantation, FSH levels remained high (between 30 and 50 mIU/mL), and E2 values were between 15 and 30 pg/mL. Six years after reimplantation, the patient became amenorrheic and FSH, LH, and E2 returned to castrated levels. The duration of efficient ovarian activity was thus estimated to be around 5 years. The child, now 6 years old, is in perfect health and thriving.
Patient 2 (JD). In 2001, at the age of 17, the patient presented with a neurectodermic tumor of the right orbit. She underwent surgery and radiotherapy. Because of the presence of chest metastasis, she received chemotherapy (etoposide, ifosfamide, vincristine, and doxorubicin) prior to hematopoietic stem cell transplantation. Before chemotherapy, ovarian tissue was cryopreserved.Two large biopsies (10 X 5 mm) were taken from each ovary, cut into small cubes (2 mm in size), and stored in liquid nitrogen after slow-freezing. Histology of a small cube revealed a very high follicular density (> 50 primordial follicles/mm3).The patient became amenorrheic after chemotherapy, with FSH and E2 values at castrated levels.
In 2008, the patient expressed a desire to conceive. She underwent reimplantation of cryopreserved ovarian tissue after decortication of her atrophic ovaries, according to the technique described by Donnez et al. (14,29). Serial sections of removed cortex failed to demonstrate the presence of any follicles. The frozen-thawed ovarian pieces were placed on the decorticated area and covered with Interceed (Johnson and Johnson, Raritan, USA) (Figure 2).
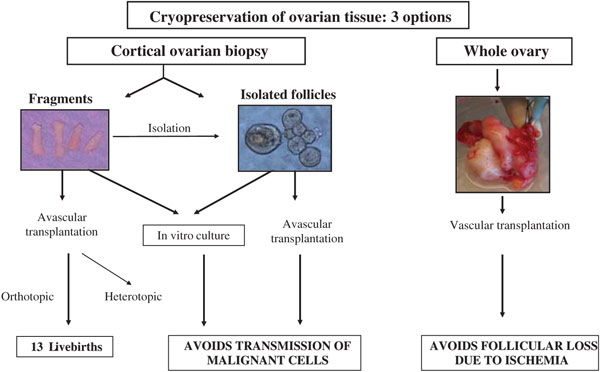
Figure 1: Options for ovarian tissue cryopreservation and reimplantation.
Three-and-a-half months later, a first E2 peak was detected, with a concomitant decline in FSH. Ultrasonography revealed the development of a follicle with each cycle. FSH and LH levels were less than 10 mIU/mL. Restoration of consecutive menstrual bleeding was observed, and the patient was encouraged to have regular sexual intercourse. Nine months after grafting, the patient experienced amenorrhea. Her HCG test was positive, and vaginal ultrasound revealed a viable intrauterine pregnancy. The triple test and nuchal thickness were normal. After an uneventful pregnancy, in April 2010, she delivered a healthy boy weighing 2.830 kg at 38 1/2 weeks of gestation.
Patient 3 (DM). In 2001, a 28-year-old woman was diagnosed with primary mediastinal B cell non-Hodgkin’s lymphoma. A first-line chemotherapy protocol was administered weekly (induction chemotherapy, VACOP-B: etoposide, doxorubicin, cyclophosphamide, vincristine, bleomycin) for 12 weeks. A relapse occurred 6 months after completion of therapy, and a second-line chemotherapy (MINE-ESHAP: mesna, ifosfamide, mitoxantrone, etoposide, Ara-C, cisplatin) was then administered, followed by high-dose chemotherapy (BEAM: BCNU, etoposide, Ara-C, and melphalan) with autologous stem cell support 7 weeks later. Ovarian tissue was cryopreserved after second-line protocol prior to high-dose chemotherapy. The patient experienced amenorrhea related to POF. FSH values were high (40–104 IU/L), and anti-Müllerian hormone (AMH) and inhibin B levels were undetectable.
In 2003, four of seven stored pieces of ovarian tissue were thawed and reimplanted by laparotomy, according to the technique described by Meirow et al. (21,30).
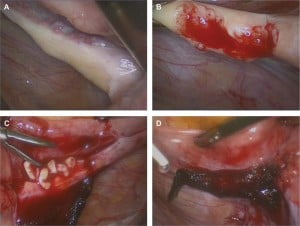
The ovaries were atrophic; no follicles were observed in the ovarian biopsy taken.
From 6 months after reimplantation, ovarian activity was proved by vaginal ultrasound and high E2 levels. AMH levels that had been undetectable during the first five post-transplantation months started to rise in the sixth month, and were found to be high (5.3 ng/mL) by month eight. The decrease in FSH is shown in Figure 3, with levels remaining over 30 mIU/mL until month six.
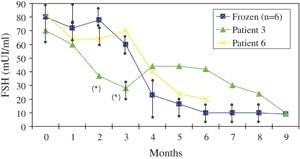
Nine months after reimplantation, spontaneous follicle development was observed by ultrasonography. Basal hormone measurements on day four of the ninth post-transplantation month showed an FSH value of 7.9 IU/L and LH of 6.8 IU/L when follicle size reached 15 mm. Mild stimulation with gonadotropins was initiated and GnRH antagonist was given, according to a modified natural cycle protocol (31). When follicle size reached 18–20 mm, HCG was added, and follicle aspiration was performed 34–36 hours later.
A single mature egg was retrieved and fertilized with the husband’s spermin vitro. On day two post-fertilization, a four-cell embryo was transferred to the uterus, which led to pregnancy.
At 38 weeks and five days of gestation, a healthy female infant weighing 3.000 kg was delivered by cesarean section (21,30).
From 3 to 7 months after delivery, the patient experienced four spontaneous menses, and a spontaneous pregnancy was diagnosed by sonography. The pregnancy, however, resulted in spontaneous abortion. At 5 months after delivery, her hormone profile showed FSH at 83 IU/L, LH at 61 IU/L, and E2 at 41 pg/mL, indicating that restoration of ovarian activity did not exceed 2 years.
Patient 4 (ID). The patient was suffering from stage IV Hodgkin’s disease
at age 24. After one regimen of ABVD therapy (doxorubicin, bleomycin, vincristine, dacarbazine), the right ovary was removed by laparoscopy for ovarian tissue cryopreservation. The patient then received five further courses of ABVD therapy but rapidly relapsed. Autologous bone-marrow transplantation (BMT) was undertaken in November 2000.The conditioning regimen included high-dose chemotherapy: three EVA courses (etoposide, vinblastine, doxorubicin) and a CBV conditioning regimen (cyclophosphamide, carmustine, etoposide). After BMT, the patient was considered to be disease-free but never recovered spontaneous cycles. In 2002, blood tests showed menopausal hormone values (FSH 114 mUI/mL), and hormone replacement therapy was initiated.
In 2004, the patient stopped treatment in an attempt to conceive, but castration was confirmed (inhibin B < 15; FSH 128 mIU/mL). She underwent a first orthotopic transplantation of frozen-thawed ovarian tissue in two steps, 32 months after the end of chemotherapy. During a first laparoscopy, a window was created in the pelvic peritoneum of the ovarian fossa, and the left atrophic ovary was incised longitudinally. Ovarian biopsy confirmed the absence of any remaining follicles in the orthotopic ovaries. A second laparoscopy was performed 1 week later, and both peritoneal and ovarian sites appeared to be well vascularized. Three fragments were sutured inside the ovarian incision, nine inside the peritoneal pocket, and six in a right abdominal subcutaneous site.
Four months later, follicular development was observed in all sites, and hormone values showed ovarian function restoration (E2 > 100 pg/mL and FSH < 10 mIU/mL).
Eight months after this first reimplantation, the patient experienced an ovulatory cycle (day 12: LH 36 mIU/mL; E2 410 pg/mL; follicle size 19.5 mm) resulting in an intrauterine pregnancy, but unfortunately ending in miscarriage at seven weeks’ gestation.
One year later, FSH values over 30 mIU/mL were sometimes observed during the menstrual phase, but ovarian activity was still present (E2 > 200 pg/mL).This progressive increase in FSH concentrations and decrease in inhibin B levels suggested depletion of the graft follicular reserve. A second transplantation was therefore carried out in May 2006. By two-step laparoscopy, two ovarian fragments were sutured to the remaining ovary, and two were placed in a left abdominal subcutaneous site.
During the fifth spontaneous cycle following the second transplantation procedure, two follicles of 15 mm in diameter each were observed at the ovarian site at the time of ovulation. A positive human chorionic gonadotropin level was detected at day 14 of the luteal phase, and a clinical pregnancy was later confirmed by vaginal ultrasound. A normal healthy baby girl (3.130 kg) was born (22).
Thirty-three months after the second reimplantation, the patient experienced a third intrauterine pregnancy and gave birth to a healthy girl (2.870 kg) at 39 weeks.Three months after delivery, the patient recovered normal menstrual cycles and requested contraceptive treatment.
After the case recently published by Ernst et al. (24), this is the second woman to have delivered twice after an ovarian transplant.
Patient 5 (CA). At the age of 27 years, the patient was diagnosed with Ewing’s sarcoma, which developed from the right seventh and eighth ribs. She only had her right ovary at the time of ovarian tissue cryopreservation, since the left ovary had already been removed due to a dermoid cyst involving the whole ovary. Prior to any gonadotoxic treatment, part of the right ovary (approximately one-third) was retrieved in 2004. The tissue was transported on ice for 5 hours and cryopreserved using ethylene glycol (EG) as a cryoprotectant, as previously described (23). The patient initially received six courses of VIDE (vincristine, ifosfamide, doxorubicin, etoposide) therapy, after which the remaining tumor was surgically removed together with the two affected ribs. Her chemotherapy continued with three further VAI regimens (vincristine, actinomycin D, ifosfamide). Thereafter, her menstrual cycles stopped, post-menopausal gonadotropin levels were recorded, and hot flashes developed (FSH > 80 mIU/mL).
Transplantation was performed in 2005, 32 months after the end of chemotherapy. Six pieces of cortex were thawed and sutured to the ovarian medulla of the remaining ovary. A biopsy obtained from the ovary revealed no remaining follicles upon histological examination. Four months after transplantation, gonadotropin levels gradually returned to premenopausal values, and E2 levels increased. During her second menstrual cycle, the patient received 150 IU rec-FSH (Puregon, Organon, Skovlunde, Denmark) per day, starting on day one and continuing up to the day of ovulation, induced by 10,000 IU HCG (Organon, Skovlunde, Denmark). Two oocytes were recovered. One three-cell embryo was transferred, resulting in an intrauterine pregnancy. She delivered a healthy girl weighing 3.204 kg by cesarean section at 39 weeks (23).
The patient breast-fed until October 2007 and returned to the fertility clinic in January 2008 for further IVF treatment. However, a pregnancy test revealed that she had already conceived naturally without any treatment. The gestational age was seven weeks (+ 4), evaluated by transvaginal ultrasonography. After an uneventful pregnancy, the patient delivered a second healthy girl on 23 September 2008, weighing 3.828 kg and measuring 54 cm in length.
This is the first woman to have given birth twice after transplantation of frozen-thawed ovarian tissue (23,24).
Patient 6 (CA). The patient was diagnosed with Hodgkin’s lymphoma in 2001 at 25 years of age. She received six regimens of ABVD treatment and supradiaphragmic radiotherapy. In September 2003, at the age of 27 years, she relapsed, and ovarian tissue was collected for freezing before treatment with three regimens of DHAP and autologous BMT.
The first reimplantation was performed 18 months after cryopreservation by longitudinal incision into the ovarian cortex, creating two pockets, one on either side of the ovary. Ten frozen-thawed fragments were sutured to the ovarian medulla. Restoration of ovarian activity was proved 5 1/2 months after reimplantation.
Although her ovarian tissue was still functional after this first transplantation, the patient returned for grafting of additional ovarian cortex in order to increase the follicular pool and possibly improve her chances of conceiving. Thus, 15 months after the first transplantation, another 12 pieces of cortex were transplanted. Five months after the second reimplantation, her FSH level was 16.5 mIU/mL and E2 200 pg/mL.
The patient then experienced uterine adenomyosis which, after 2 months of pituitary down-regulation with a GnRH agonist, was removed during an operation 7 months after the second autotransplantation using the transverse H incision technique (33). After this surgery, she underwent one treatment cycle with assisted reproduction (stimulation with 150 IU rec-FSH, like patient 5), resulting in retrieval of one mature oocyte and transfer of one four-cell embryo, which subsequently implanted.
Following a normal uneventful pregnancy, the patient developed slight pre-eclampsia close to term and delivered a healthy boy weighing 2.600 kg and measuring 50 cm in length at 37 weeks by cesarean section in 2008 (23).
Patient 7 (SJS). The patient was diagnosed with stage IIIb Hodgkin’s disease at 20 years of age. Prior to chemotherapy, ovarian cortex was removed and cryopreserved according to Gosden’s protocol (19). The patient then received several rounds of chemotherapy (doxorubicin, bleomycin, vincristine, dacarbazine, and cisplatin), followed by liposomal doxorubicin and gemcitabine. Over the next 3 years, she suffered two relapses and was finally treated by BMT.
She subsequently experienced amenorrhea related to ovarian insufficiency, as proved by FSH, LH, and E2 levels (FSH 62.7 mIU/mL; LH 33.7 mIU/mL; E2 23 pg/mL).
She underwent ovarian cortex reimplantation according to the technique previously described by Silber et al. (32), and, 3 1/2 months later, ovarian activity was detected.
Seven months after reimplantation, her FSH level was 2.3 mIU/mL, LH 1.9 mIU/mL, and E2 92 pg/mL. Eight months after reimplantation, the patient became pregnant naturally. She delivered a healthy boy weighing 3.089 kg after 38 weeks of gestation.
Patient 8 (PP). In 1999, a 27-year-old woman presented with microscopic polyangiitis (MPA) that was antineutrophil cytoplasmic antibody (ANCA)-positive and myeloperoxidase (MPO)-positive.
Clinical signs included diffuse renal, skin, digestive organ, and nervous system involvement.
After 2.55 g of cyclophosphamide, one ovary was removed and cryopreserved. The ovarian tissue was frozen using the slow-cooling technique and DMSO as a cryoprotectant. Seventeen ovarian cortical fragments, each approximately 8 X 8 mm in size, and three of 2 X 2 mm, were cryopreserved. Over the next 2 years, the patient received 7.65 g of cyclophosphamide i.v. and 58 g per os. In 2003, she stopped taking the combined estrogen-progestin pill. She then showed clinical signs of menopause with high plasma levels of FSH (234.8 IU/L) and LH (97.7 IU/L).
Eight-and-a-half years after cryopreservation, the patient wished to conceive. During a first laparoscopy, one 8 X 8 mm fragment was thawed, cut into small fragments, and placed in a peritoneal window and on the ovarian medulla. Three days later, a second laparoscopy was performed, during which eight thawed ovarian fragments were placed in left and right peritoneal windows, as well as on the left ovarian medulla (26).
Four months after the autografting procedure, a decrease in serum gonadotropin levels and an increase in inhibin and E2values were noted. Five-and-a-half months later, a decrease in FSH (10 mIU/ mL) was observed, with inhibin B and AMH levels of 25 pg/mL and 1.5 ng/mL, respectively.
The patient underwent stimulation with rec-FSH (225 IU) associated with GnRH antagonist.
In the second month of stimulation, two embryos were transferred, but the patient suffered an ectopic pregnancy. In the fourth month of stimulation (14 months after reimplantation), one embryo was transferred, which resulted in an intrauterine pregnancy.
At 37 gestational weeks, the patient developed pre-eclampsia, so induction of labor was decided upon. She gave birth to a 2.030 kg healthy girl by vaginal delivery. Twenty-four hours later, she developed HELLP syndrome, which resolved in 3 days (26).
Patient 9 (AP). In 2006, the patient was 36 years of age and had experienced 2 years of primary infertility before being diagnosed with breast cancer. She underwent quadrantectomy and axillary node dissection (pT1 N1/11 M0 and triple-negative, i.e. negative for estrogen, progesterone, and HER2 receptors) and, just before chemotherapy, right ovarian cortex removal by laparoscopy. She subsequently received 3 cycles of fluorouracil, epirubicin, and cyclophosphamide (FEC) plus 3 cycles of docetaxel, followed by 25 standard radiotherapy sessions. She was amenorrheic from her third FEC cycle, and her serum FSH and AMH levels were, respectively, 36.3 mIU/mL and 0.6 ng/mL (normal range > 1 ng/mL).
Ovarian tissue transplantation went ahead 20 months after the end of chemotherapy. Two cortical strips of approximately 3 cm2 were grafted. On day 63 after transplantation of frozen-thawed cortical strips to the left medulla, the patient experienced spontaneous menses, at which point her serum FSH and AMH levels were 10.4 mIU/mL and 1.8 ng/mL, respectively.
Between May and November 2008, four cycles of controlled ovarian stimulation (COS) were performed with recombinant FSH (27). At the first attempt, four oocytes were obtained, only two of which were mature. One of these failed to fertilize and the other degenerated after ICSI. In the three subsequent COS cycles, 5 mg per day of letrozole (Femara, Novartis Laboratories, Madrid, Spain) was given from days 1 to 5 of the menstrual cycle. Gonadotropin administration (225 IU) was initiated on day two at the dose stipulated above. Daily injections of GnRH antagonist (Cetrotide, Merck-Serono, Paris, France) were added when follicles reached 14 mm, and HCG when follicles measured 18–19 mm. In the two first cycles, respectively five and four mature oocytes were obtained and vitrified using the previously described Cryotop method (34). At the last attempt, five metaphase-II oocytes were obtained with the same COS protocol. All 14 oocytes, both fresh and vitrified-warmed, were microinjected.
On day three, two good-quality embryos were replaced, two were found to be blocked, and the remaining three were maintained in culture for further observation, but did not reach the blastocyst stage. Embryo replacement took place on day 335 after transplantation. The patient was confirmed pregnant with twins and, after an uneventful pregnancy, spontaneous rupture of the membranes occurred at week 33 and 2 days. Two boys weighing 1.650 kg and 1.830 kg, both Apgar 9/10, were delivered by cesarean section and discharged from the neonatal unit in good health 19 days later (27).
Patient 10 (PP). In 2005, a 20-year-old woman suffering from homozygous sickle cell anemia presented with cerebrovascular stroke and underwent monthly exchange transfusions. BMT with an HLA-identical sibling donor was offered to the patient the same year. Right oophorectomy was performed by laparoscopy. Eight ovarian cortical fragments (1 cm/0.5 cm) were cryopreserved according to Gosden’s protocol (19).
After ovarian cryopreservation, the patient received a conditioning regimen with busulfan (12.8 mg/kg total dose) and cyclophosphamide (200 mg/ kg total dose) (26), and then 2.4 X 108 nucleated cells/kg from her HLA-genoidentical brother, who was heterozygous for hemoglobin S. After BMT, she presented with amenorrhea related to POF (FSH > 90 mIU/mL) (28).
Eighteen months after BMT, the patient wished to become pregnant. A two-step orthotopic autotransplantation procedure was therefore performed by laparoscopy in April 2008, according to the technique described by Donnez et al. (20) and modified by Piver et al. (26). The first step involved triggering local inflammation and inducing neoangiogenesis. Small ovarian cortical strips were placed to facilitate production of angiogenic factors, such as vascular endothelial growth factor. One thawed strip of ovarian cortex was cut into six fragments. One fragment was sutured inside the ovarian incision, while the other five were deposited in a peritoneal window. In a second step performed 3 days later, three thawed cortical strips were fixed to the left ovary and one inside the peritoneal window.
Four months after transplantation, vaginal echography carried out for abdominal pain revealed follicular development in both transplanted sites. Two follicles were present in the left ovary and two more in the peritoneal window, one resembling an initial corpus luteum.
Normalization of FSH values and significant AMH levels were observed 19 weeks after grafting.
At 38 weeks of gestation, the patient gave birth to a healthy girl by cesarean section, heterozygous for sickle cell anemia, weighing 3.700 kg (28).
Fresh ovarian tissue reimplantation
For discussion purposes, a comparison was made with patients undergoing fresh ovarian tissue reimplantation, who subsequently became pregnant. All patients were operated on by SJS.
To date, pregnancy has been achieved in seven pairs of female monozygotic twins, involving one sister with idiopathic POF (25,32,35,36). In each case, the donor underwent unilateral oophorectomy by laparoscopy or mini-laparotomy under general anesthesia. One-quarter to one-third of the donor ovary was transplanted as a cortical slice, the remaining tissue being cryopreserved as back-up for the primary graft, if necessary. In total, seven babies have been born to seven women from fresh cortical grafts, with one woman currently pregnant with healthy twins. One of the patients who conceived after transplantation of fresh tissue from her sister again developed POF after delivery. She underwent further grafting of ovarian tissue that had been cryopreserved at the time of fresh tissue reimplantation. Four months later, restoration of ovarian activity was observed. The patient became pregnant and delivered a healthy baby girl weighing 4.111 kg at 40 weeks of gestation.
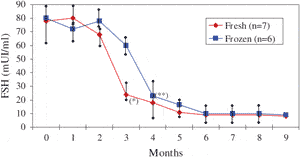
Restoration of ovarian activity was evidenced in all seven cases approximately 3 months after reimplantation by the significant decrease (P < 0.05) in FSH, compared to preoperative values (Figure 4). Serum FSH levels on day three of cycles had begun to decline by 3 months after surgery in all cases and had reached normal base-line values in every recipient by 5 months. This decline in FSH values after fresh tissue reimplantation is shown in Figure 4.
For this reason, procedures to preserve fertility are commonly implemented as an integral part of treatment. Various strategies of fertility preservation are applied depending on the risks and probabilities of gonadal failure, the patient’s general health at diagnosis, and the partner status (1–8).These include IVF and embryo cryopreservation, which are standard established procedures with predictable and well documented outcomes in terms of pregnancy and childbirth rates (4–6).
Harvesting and cryopreservation of ovarian tissue before commencing sterilizing chemotherapy has been increasingly practiced during the past decade. With ovarian cryobanking, abundant primordial follicles, containing small, less differentiated oocytes, are stored (3–6).
Ovarian tissue has been successfully cryopreserved and reimplanted into humans on numerous occasions to date. Despite very encouraging results reported in recent years (20–28), which are actually much better than the first IVF results published more than 30 years ago, the procedure remains controversial as it does not appear in the official guidelines of the American Society for Reproductive Medicine (ASRM).
In the present study, the first 13 births after orthotopic frozen-thawed ovarian cortex reimplantation are reported and analyzed. Restoration of ovarian activity in this group is compared with the series of seven births in the fresh tissue group published by Silber et al. (25,32,35,36).
Restoration of ovarian activity
Restoration of ovarian function was observed in all cases of orthotopic frozen-thawed ovarian cortex reimplantation. In all instances, it took between 3 1/2 and 6 1/2 months after reimplantation before a rise in E2 and a decrease in FSH were detected. The time interval between implantation of cortical tissue and the first E2 peak is also consistent with data obtained from sheep (37) and human beings (4,6,12), although some variation may be observed. Such variation could be explained by a difference in follicular reserve at the time of cryopreservation, some women having already received a first regimen of chemotherapy before ovarian biopsy and cryopreservation. Indeed, the time to the E2 peak, concomitant with the FSH decrease, was longer (5 1/2 to 6 1/2 months) in patients who had received chemotherapy before cryopreservation than in those who had not (3 1/2 to 4 1/2 months) (Table I). In cases published by Meirow et al. (21,38) and Andersen et al. (24), the decline in FSH values was much slower, demonstrating the probably deleterious effect of previous chemotherapy on vascularization. Meirow et al. recently demonstrated the detrimental effect of chemotherapy on the vascular network of grafts (39). Their findings may explain not only ovarian reserve depletion but also the delay that occurs between reimplantation and revascularization of the graft, as proved by delayed ovarian activity restoration (40). Indeed, recent studies in our group have clearly shown that revascularization of grafts (in a human xenograft model) depends not only on neoangiogenesis from the host but also on existent blood vessels in grafted tissue. Moreover, the presence of chimeric vessels in transplants after 1 week highlighted the crucial role of the pre-existing vascular network in grafts at the time of reimplantation (41).
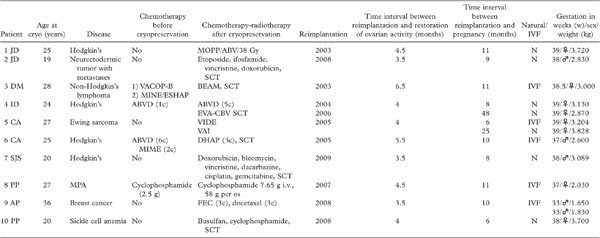
MPA = microscopic polyangiitis; N = naturally pregnant (by sexual intercourse); SCT = stem cell transplantation.
In a comparison of both frozen-thawed and fresh transplantation groups (25,32,35,36), the decline in FSH values was found to be faster after fresh tissue reimplantation than frozen-thawed tissue grafting (Figure 4). Indeed, a significant (P < 0.05) decrease in FSH was observed after 3 months in the fresh tissue group compared to 4 months in the frozen tissue group. From the fourth month, however, FSH values were similar in both groups.
During the first months of ovarian restoration, it was not uncommon to observe relatively high FSH (< 20 mIU/mL) and E2levels on days 3–5, demonstrating a poor follicular reserve. It should be noted that pregnancy was only obtained in cases of normal cycle length and day three FSH < 10 mIU/mL and E2 < 30 pg/mL (cases JD, ID, AP). This type of cycle was generally observed from 8 months after transplantation.
The duration of restored ovarian activity was also shorter (1 to 2 years) in the subgroup of women who had received chemotherapy before cryopreservation, compared to those who had not (4 years). Indeed, in some patients (DM, CA), the duration of graft activity was less than 2 years, while in others, who had not received chemotherapy, it was considerably longer (4 years). These data also strongly suggest that a first regimen of chemotherapy could have a deleterious effect on the ovarian reserve.
The age of patients at the time of cryopreservation is also a predictive factor. Indeed, all except one (AP) were under 30 years of age (17–28 y), and six of the ten patients were ≤ 25 years (mean 23 y; range 19–25 y).
From the present series, some preliminary conclusions can be drawn with respect to the surgical techniques implemented:
In the reported series, the peritoneal window created close to the ovarian hilus (JD, ID, PP) and the ovarian medulla both appear to be equally efficient sites of reimplantation, at least for restoration of ovarian activity (as proved by follicular development). Although Demeestere et al. considered follicular activity in the peritoneal site to be lower than that observed on the ovarian medulla, others demonstrated comparable follicular activity in both locations (13–17,22,26,29).
Large strips (8–10 mm X 5 mm) and small cubes (2 mm X 2 mm) of tissue were both shown to effectively restore ovarian endocrine function.
Pregnancy rates
Some prognostic factors in terms of pregnancy rates can be determined from the observations made in this series:
1) Among the 13 live births, 8 were girls and 5 boys. All the babies were in good health. All singleton deliveries occurred after 37 weeks of gestation, and only one woman experienced HELLP syndrome after delivery. One twin pregnancy ended in delivery of two boys at 33 weeks, following rupture of the amniotic membrane.
2) The majority (6/10, 60%) of the pregnant women had not received any chemotherapy prior to cryopreservation.Two of the ten patients (20%) had received a full regimen of chemotherapy (ABVD-MINE/VACOP, MINE-ESHAP) before their ovarian tissue was cryopreserved. Among the last two patients, one had received only one line of ABVD therapy and one 2.5 g of cyclophosphamide. In all cases, especially in the four patients who had already received chemotherapy before ovarian tissue cryopreservation, histology at the time of cryopreservation showed the presence of numerous primordial follicles. This is why transplantation went ahead. Nevertheless, as proved by another study (13), the ovarian reserve was probably diminished in patients who had already undergone chemotherapy prior to ovarian tissue cryopreservation, as the life-span of the graft was shorter than in those who had not. There is no doubt here about the recovery of function of the native ovary, as that tissue received complete chemotherapeutic treatment.
In the two patients who received a full regimen (VACOP-B/ABVD-MINE) and the two who received smaller doses, histology of their ovarian biopsy at the time of cryopreservation revealed numerous primordial follicles. But it is impossible that the native ovary could have recovered its function, because biopsies from the native atrophic ovary (at the time of reimplantation) failed to reveal the presence of any follicles at all. Moreover, the native ovary was subsequently subjected to sterilizing pre-stem cell transplantation (SCT) treatment.
3) The peritoneal window created close to the ovarian hilus proved effective in cases described by Donnez et al. (4), Demeestere et al. (22), Piver et al. (26), and Roux et al. (28), but it should be emphasized that, in these four cases, laparoscopy was performed a few days (three to seven) before reimplantation in order to stimulate angiogenesis in this site prior to grafting.
In the other cases, tissue was grafted to the medulla, as previously described by Donnez et al. (13) and Silber et al. (35), and it proved equally effective, as patients also became pregnant naturally. The advantage of this technique is that it does not require two procedures. Although it has been shown that chemotherapy causes damage to ovarian blood vessels by hyalinization and lumen narrowing, neovascularization with CD34 proliferation has been concomitantly demonstrated (39). The angiogenic potential of the ovary probably explains why the ovarian medulla is also a good grafting site, even after several years of atrophy.
4) The fact that more than 50% of women were able to conceive naturally constitutes a good argument in favor of orthotopic reimplantation. Some patients required IVF to become pregnant, but it should be pointed out that in series published by Andersen et al. (23), Dolmans et al. (42), and Meirow et al. (21), an empty follicle rate as high as 29%–35% was observed during the IVF procedure after stimulation by gonadotropins. A possible cause of this high rate of empty follicle syndrome is dysfunctional folliculogenesis, whereby early oocyte atresia occurs with an apparently normal hormonal response (42). This dysfunctional folliculogenesis could be due to asynchrony between granulosa cells and oocyte maturation, as reported in human ovarian xenotransplants by Donnez’s group, the granulosa cells being mature, while the oocytes are immature (43,44). There is no doubt that the percentage of abnormal oocytes (immature or degenerated) was much higher (37%) in frozen-thawed transplanted tissue than in the general population undergoing ICSI (42). The oocyte itself could be damaged by freezing and thawing procedures, leading to a higher rate of empty follicles or oocyte alterations, while granulosa cells may be more resistant (43,44).
Another cause of damage to oocytes is the delay that occurs before efficient revascularization of the graft is achieved after transplantation (40,41). This interval was estimated to be 5 days using reoxygenation evaluation of grafts by electron paramagnetic resonance. Different factors influencing the revascularization rate should be further explored, as very recently suggested (5,41).
Risks of reimplantation
One of the most important ethical issues to consider is ensuring that the intervention does not harm the patient by dangerously delaying cancer treatment and that no remnant cells are reintroduced by subsequent transplantation (4,6). Cancer treatment takes priority over potential restoration of fertility, but offering the chance to preserve fertility may greatly enhance quality of life for cancer survivors. In the present series, no patients experienced disease recurrence (Table I).
Transmission of lymphoma via grafts of ovarian tissue from diseased donor mice to healthy recipients was reported by Shaw et al. (45,46).This study highlighted the risks of clinical transplantation of ovarian biopsy samples to women recovering from cancer, especially a blood-borne malignancy (45,46). There are certain circumstances where the risk of cancerous involvement of the ovary is absent or minimal (20) and where autografting would present little or no danger (47–49). In diseases like leukemia, however, the risk is real, and the high prevalence of minimal residual disease (MRD) in cryopreserved ovarian tissue from women with this pathology has recently been evidenced (50–52). In a very recent paper, References
Dolmans et al. highlighted the prevalence of MRD in cryopreserved ovarian tissue from chronic myeloid leukemia and acute lymphoblastic leukemia patients, which was, respectively, 33% and 70% (50).
Screening methods must be developed to eliminate the risk of cancer cell transmission with reimplantation. In some diseases, like leukemia, other options must be considered after ovarian tissue thawing, such as isolated follicle transplantation or IVM (10,11,18,53). IVM of immature primordial follicles enclosed in ovarian tissue shows real promise in this context, as new culture techniques and tissue engineering solutions for maintaining the follicular unit in culture are constantly evolving (54). Indeed, bovine primordial follicles were shown to develop and grow over 15 days in culture (55), and primate secondary follicles developed to the antral stage in a 3D culture system (56).
Conclusion
More than one-third of young women exposed to chemotherapy develop ovarian failure (2,3). In 2010, we believe it is our ethical and moral responsibility to propose cryopreservation of ovarian tissue to all adolescents and young women under IRB protocols having to undergo chemotherapy with alkylating agents.
We accept that ovarian tissue cryopreservation is a more innovative and invasive procedure than sperm cryopreservation and that all possible applications in adolescents and young women are ethically complex (57). However, based on our review of all live births to date, we believe that ovarian cortex cryopreservation, associated or not with cryopreservation of immature oocytes, should be offered before gonadotoxic chemotherapy in all cases where there is a high risk of POF and where emergency IVF is not possible.
Acknowledgements
All authors contributed equally in terms of providing individual case descriptions. J Donnez initiated the project and wrote the manuscript with M-M Dolmans.
J Donnez thanks J Squifflet and P Jadoul; S J Silber thanks M Katajima; I Demeestere thanks P Simon; D Meirow thanks J Dor; A Pellicer thanks M Sánchez-Serrano and J Crespo; C Andersen thanks E Ernst; and P Piver thanks C Roux. The authors thank Mira Hryniuk for reviewing the English grammar and syntax of the manuscript.
References
- Meirow D, Lewis H, Nugent D, Epstein N. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14:1903–7.
- Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–44.
- Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–18.
- Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–35.
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation.Hum Reprod Update. 2009;15:649–65.
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11.
- Lee SJ; ASCO Fertility Preservation Guidelines Committee. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:2680; author reply 2682–3.
- Chian RC, Gülekli B, Buckett WM, Tan SL. Pregnancy and delivery after cryopreservation of zygotes produced by in vitro matured oocytes retrieved from a woman with polycystic ovarian syndrome. Hum Reprod. 2001;16:1700–2.
- Borini A, Sciajno R, Bianchi V, Sereni E, Flamigni C, Coticchio G. Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod. 2006;21:512–7.
- Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–15.
- Amorim CA,Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–9.
- Donnez J, Bassil S. Indications for cryopreservation of ovarian tissue. Hum Reprod. 1998;4:248–59.
- Donnez J, Squifflet J, Van Eyck AS, Demylle D, Jadoul P, Van Langendonckt A, et al. Restoration of ovarian function in orthopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod Biomed Online. 2008;16: 694–704.
- Donnez J, Dolmans MM. Cryopreservation of ovarian tissue: an overview. Minerva Med. 2009;100:401–13.
- Donnez J, Jadoul P, Squifflet J,Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynecol. 2010;24:87–100.
- Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Semin Reprod Med. 2009;27:465–71.
- Arav A, Gavish Z, Elami A, Natan Y, Revel A, Silber S, et al. Ovarian function 6 years after cryopreservation and transplantation of whole sheep ovaries. Reprod Biomed Online. 2010;20:48–52.
- Dolmans MM, Martinez-Madrid B, Gadisseux E, Van Langendonckt A, Camboni A, Coupe A, et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134:253–62.
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at –196°C. Hum Reprod. 1994;9:597–603.
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21.
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–42.
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–72.
- Ernst E, Bergholdt S, Jorgensen JS, Andersen CA. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1.
- Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23: 1531–7.
- Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. In: 25th Annual Meeting of ESHRE, 28 June–1 July, 2009. Amsterdam, the Netherlands: Oxford University Press, Hum Reprod. 2009:i15.
- Sánchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simón C, et al.Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93:268.e11–3.
- Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93:2413.e15–9.
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Restoration of ovarian function after orthotopic (intraovarian and periovarian) transplantation of cryopreserved ovarian tissue in a woman treated by bone marrow transplantation for sickle cell anaemia: case report. Hum Reprod. 2006;21:183–8.
- Meirow D, Ben Yehuda D, Prus D, Poliack A, Schenker JG, Rachmilewitz EA, et al. Ovarian tissue banking in patients with Hodgkin’s disease: is it safe? Fertil Steril. 1998;69: 996–8.
- Elizur SE, Aslan D, Shulman A, Weisz B, Bider D, Dor J. Modified natural cycle using GnRH antagonist can be an optional treatment in poor responders undergoing IVF. J Assist Reprod Genet. 2005;22:75–9.
- Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63.
- Fujishita A, Masuzaki H, Khan KN, Kitajima M, Ishimaru T. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132–8.
- Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remoh J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–64.
- Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–6.
- Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–8.
- Baird DT, Campbell BK, Souza C, Telfer EE. Long-term ovarian function in sheep after ovariectomy and autotransplantation of cryopreserved cortical strips. Eur J Obstet Gynecol Reprod Biol. 2004;113:55–9.
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril. 2007;87:418e7–15.
- Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22:1626–33.
- Van Eyck AS, Jordan B, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–81.
- Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85.
- Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24:2778–87.
- Nottola S, Camboni A, Van Langendonckt A, Demylle D, Macchiarelli G, Dolmans MM, et al. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril. 2008;90:23–32.
- Camboni A, Martinez-Madrid B, Dolmans MM, Van Langendonckt A. Autotransplantation of frozen-thawed ovarian tissue in a young woman: ultrastructure and viability of grafted tissue. Fertil Steril. 2008;90:1215–8.
- Shaw JM, Bowles S, Koopman P, Wood EC, Trounson OA. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996;11:1668–73.
- Shaw JM, Trounson AO. Oncological implications in the replacement of ovarian tissue. Hum Reprod. 1997;12: 403–5.
- Gosden RG, Rutherford AJ, Norfolk DR. Transmission of malignant cells in ovarian grafts. Comments. Hum Reprod. 1997;12:403.
- Moomjy M, Rosenwaks Z. Ovarian tissue cryopreservation: the time is now. Transplantation or in vitro maturation: the time awaits. Fertil Steril. 1998;69:999–1000.
- Kim SS, Radford J, Harris M, Varley J, Rutherford AJ, Lieberman B, et al. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod. 2001;16:2056–60.
- Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is not safe. Blood. 2010;116:2908–14.
- Rosendahl M, Andersen MT, Ralfkiær E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukaemia. Fertil Steril. 2010;94:2186–90.
- Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–13.
- Dolmans MM, Yuan WY, Camboni A, Torre A, Van Langendonckt A, Martinez-Madrid B, et al. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online. 2008;16:705–11.
- Desai N, Alex A, Abdelhafez F, Calabro A, Goldfarb J, Fleischman A, et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119.
- McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 2010; 139:971–8.
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–97.
- Jadoul P, Dolmans M, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16:617–30.
