David Miller, Ph.D.a Jonathan Summers, Ph.D.b and Sherman Silberc
a Reproduction and Early Development Group, Department of Paediatrics, Obstetrics and Gynaecology, University of Leeds, b School of Mechanical Engineering, University of Leeds and c Infertility Center of St. Louis, St. Luke’s Hospital, St. Louis, Missouri
Fertility and Sterility, April 1, 2004
Abstract
This study examined the possibility that genetically based sex-determination mechanisms have evolved to ensure a balanced male/female ratio and that this temperature-independent checkpoint might have been unavailable to long-extinct reptiles, notably the dinosaurs. A review of the literature on molecular and phylogenetic relationships between modes of reproduction and sex determination in extant animals was conducted. Mammals, birds, all snakes and most lizards, amphibians, and some gonochoristic fish use specific sex-determining chromosomes or genes (genetic sex determination, GSD). Some reptiles, however, including all crocodilians studied to date, many turtle and tortoise species, and some lizards, use environmental or temperature-dependent sex determination (TSD). We show that various modes of GSD have evolved many times, independently in different orders. Animals using TSD would be at risk of rapid reproductive failure due to a skewed sex ratio favoring males in response to sustained environmental temperature change and favoring the selection of sex-determining genes. The disadvantage to the evolving male sex– determining chromosome, however, is its decay due to nonrecombination and the subsequent loss of spermatogenesis genes. Global temperature change can skew the sex ratio of TSD animals and might have played a significant role in the demise of long-extinct species, notably the dinosaurs, particularly if the temperature change resulted in a preponderance of males. Current global warming also represents a risk for extant TSD species.
Efficient sexual reproduction requires a balanced population of males and females. Mammals universally accomplish this by relying on the Y-borne, testis-determining gene, SRY, to trigger testis development (1– 6). However, the SRY gene is not found in any other genera that use genetic sex determination (GSD) (7) (e.g., birds, insects, amphibians, lizards, and snakes), and indeed GSD in unrelated animals have arisen independently many times over (7–9).
Despite highly varied “triggering” mechanisms, a variety of vertebrates share the same or similar, highly conserved downstream genes that operate in sex differentiation, which suggests that at the molecular level, the genetic programs involved in GSD and temperature-dependent sex determination (TSD) are closely related (10). This is perhaps not unexpected, given that the development of the testis and ovary and their constituent cells is broadly similar in amphibians, reptiles, mammals, and birds. The mechanisms that trigger those genes to direct testis or ovary development from the primordial gonads, however, whether GSD or TSD, are quite different. Nonetheless, these sex-determining mechanisms all have the same common goal of ensuring a balanced male/female sex ratio.
The original work of Pieau (11, 12), Yntema (13), Head (14), Bull (15–18), Wibbels and colleagues (19–22), Crews and colleagues (23–26), and Fergusson and Joanen (27) describing TSD in some groups of extant reptiles, including all crocodilians studied to date, showed that animals could equilibrate their sex ratio without the requirement for specific sex-determining chromosomes and/or genes. Unlike animals that make use of GSD, however, TSD-dependent animals rely on a more intimate and precarious relationship with their environment, whereby a balanced sex ratio is highly sensitive to and dependent on the temperature of egg incubation (28). Early reports of the exquisite temperature sensitivity of egg incubation in alligator sex determination alluded to a possible role for TSD in the extinction of ancient archosaurs, notably the dinosaurs and their relatives (14, 27, 29), assuming that those ancient animals, by virtue of their phylogenetic relationship with crocodilians, also used TSD. Arguing against this hypothesis, Rage (30) indicated that phylogenetic relationships and sex-determining mechanisms were not mutually inclusive and that the mechanism of sex determination used by these ancient reptiles could not be inferred from related “modern” species. More significantly, some extant families using TSD today also existed at the time of the great impact event of 65 MYA that probably wiped out the dinosaurs, and the survival of these animals might call into question the importance of TSD in animal extinctions in general and dinosaur extinction in particular.
Although the precise environmental effects of the great impact event of 65 million years ago (MYA) are open to debate, it is not contested that profound global environmental changes must have occurred (31–34). These changes might have exacerbated prevailing and chronic deterioration in the global environment from volcanic activity and fluctuations in the carbon cycle and put additional pressure on existing archosaurian populations. In particular, changes in global temperature would have had a more pronounced effect on TSD-dependent animals than on animals using GSD. Indeed, the concern over survival of extant TSD-dependent reptiles in the wake of contemporary global warming has recently been reiterated after empirically derived evidence that even modest temperature increases might endanger some turtle species (35).
In the absence of living specimens, we cannot know whether dinosaurs used TSD. We can, however, provide a theory of its likelihood by a two-part comparison with extant species. The first draws on conclusions based on species phylogeny and physiology. The second relies on the significant advances in our understanding of the molecular control of mammalian sex determination and the equivalent processes in reptiles. Herein, we show why GSD modes of sex determination are driven to successfully maintain a balanced sex ratio and why those animals without such a foolproof mechanism are at risk of reproductive failure due to gross skewing of the sex ratio.
CHRONOLOGIC AND PHYSIOLOGIC RELATIONSHIPS BETWEEN ANCIENT AND EXTANT ARCHOSAURS (FIG. 1)
Dinosaurs and crocodiles are members of the Archosauria, a major group of diaspsids that appeared in the Early Triassic period, some 245 MYA. By the Late Triassic period (225 MYA), the dominant representatives were dinosaurs, champosaurs, pterosaurs, and crocodilians (36). Modern birds were probably derived from avian archosaurs that first appeared during the Jurassic period and expanded their range in the Cretaceous period, during which they would have shared the skies with the dominant pterosaurs (37–39).
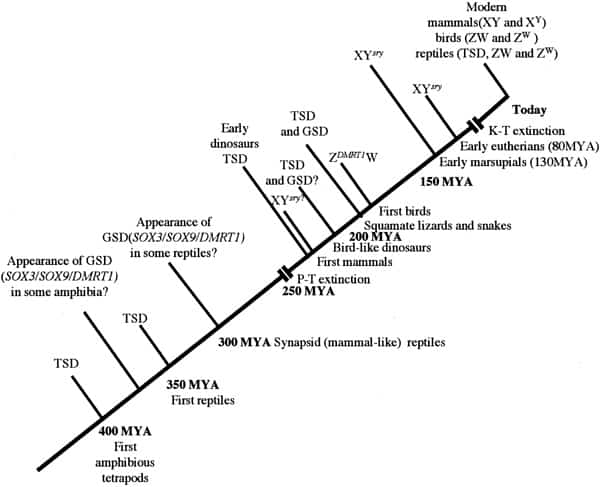
Chronology of sex determination during geologic time. In this scenario, TSD is assumed to have been the dominant form of sex determination for much of the Paleozoic era from 500 to 245 MYA. We suggest that GSD (both XY and ZW forms) evolved in some amphibian and reptilian groups (notably synapsids and sphenodonts) before the Permian extinction (P-T) event of 245 MYA. Archosaurs had probably not developed GSD at the time of their appearance in the mid-Triassic but that a ZW form had arisen in the group giving rise to the Ornithuriae. In common with amphibians and fish, these reptiles and the earliest mammals independently developed a “primitive” homomorphic set of sex chromosomes (XY or ZW) derived from ancestral autosomes. In time, the nonrecombining regions on the Y and W contributed to a process of decay and reduction that has led to markedly heteromorphic sex chromosomes in eutherians and marsupials (XY) and in carnite birds and vipers (ZW). To date, no heteromorphic sex chromosomes have been observed in amphibian or fish species that use GSD. Miller. Sex determination and dinosaur extinction. Fertil Steril 2004.
Crocodilians (TSD dependent) and avians (GSD dependent) are the only Archosaurian taxa that have persisted to this day. Assuming a post-Cretaceous–Tertiary boundary (K-T) global environmental catastrophe, crocodilians but not dinosaurs must have been able to adapt successfully to the changing environment. Perhaps physical and biological constraints did not favor adaptable modes of TSD in dinosaurs. Although there are no dinosaurs that we can use to test this idea, it should be possible to manipulate crocodilian environments, either in the laboratory or in situ, to determine whether adaptive responses capable of restoring a normal sex ratio can occur in nature (see Conclusions).
Studies on oviparity in the American alligator, Alligator mississippiensi, suggest a physiologic link between crocodilian and ancient archosaurian reproductive function. In common with birds, this species has separate uterine regions for formation of the egg membranes and calcareous layers (40), which indicates that extinct reptiles might have shared a common mode of egg production with contemporary archosaurs. Furthermore, fossil evidence based on skeletal comparisons suggests that, like modern crocodilians, dinosaur hatchlings had relatively mature perinatal pelvic girdles, an observation that is consistent with their sharing the same mobility and, by extension, similar nesting habits with modern crocodile young (41). On another tack, recent applications of spiral computerized tomography to fossil endocasts has provided compelling evidence that the Allosaur brain case was organized along lines similar to that of modern crocodilians and quite distinct from that of birds (42). Moreover, a reptilian and not avian style of lung ventilation has also been inferred from fossilized saurians, which suggests that dinosaur circulation had more in common with ectothermic crocodiles (43). Although limited, these comparisons are significant because they suggest physiologic similarities between extant reptiles and extinct dinosaurs, consistent with their shared taxonomy.
In terms of their sex determination, birds probably devel- oped GSD in parallel with their endothermy some 170 MYA. Unlike endothermy, which probably restricts TSD modes of sex determination, ectothermy puts animals at “liberty” to use either TSD or GSD, and indeed there are examples in nature of animals that occasionally use both (26, 44–46). Why, then, did other reptilian species evolve to use GSD exclusively? Because GSD modes of sex determination are immune to the environmental vicissitudes that challenge animals using TSD, one possibility is that a strong selection bias favors the adoption of GSD in environments in which a deleterious change in temperature becomes a species-threatening issue. Once it has gained a “foothold,” however, GSD might supplant TSD because of the “selfish” interest that the sex-determining gene has in assuring its own intergenerational continuance. Closer examination of the molecular mechanism of GSD in mammals shows why this is likely to be so.
MOLECULAR BASIS AND EVOLUTION OF GSD
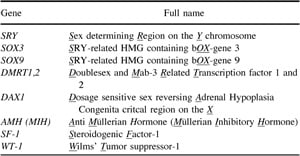
The specific chromosomes and genes responsible for GSD have evolved independently in amphibians, reptiles, birds, and mammals, thus indicating significant evolutionary advantages in its development (47). Mammals, for example, use an XX-XY, male heterogametic system from what were originally autosomes, centered around the emergence of the SRY (testis-determining) gene on the Y chromosome between 200 and 300 MYA (1, 4, 48). Insect species also developed an XX-XY male heterogametic system much earlier that has no relation to the mammalian. Birds also developed a heterogametic system (ZZ-ZW female) from what were originally autosomes (Fig. 1). Of some note is the discovery that the bird and reptile Z chromosome shares many genes in common with chromosome 9 in humans and its equivalent in other mammals, including DMRT1, a gene crucial in testis development (9).
In placental and marsupial mammals, as well as in birds and in higher snakes, the sex chromosomes are heteromorphic, meaning that one is much smaller than the other. Current evidence suggests that this is due to selective chromosomal atrophy and gene loss over many millions of years (49, 50). However, all amphibians studied to date make use of GSD with relatively large sex-determining chromosomes that are microscopically indistinguishable (homomorphic). Interestingly, amphibians using GSD can often undergo phenotypic sex reversal when eggs are incubated at higher temperatures (51), which shows that although normally dormant, TSD can be activated in GSD animals with homomorphic sex-determining chromosomes. As a group, fish also include GSD- and TSD-dependent species, with some evidence of environmentally dependent interchangeability in the mode of sex determination (45), suggestive of an intermediate or transitional state between TSD and GSD in these animals.
The inherent stability of GSD in mammals and birds is due to the continual atrophy of the sex-determining chromosome itself. The driving force for this process is selective failure of meiotic recombination between the sex chromosomes leading, over time, to the gradual degradation of the nonrecombining portion of the sex-determining chromosome (49, 50). This is most apparent in dasyurid marsupials, which have tiny Y chromosomes, fully differentiated from the X and often absent from their somatic tissues (52) and absent altogether from the mole voles, in which the Y is completely missing (53). This decay of what was previously an ordinary paired autosome has resulted in selective inactivation of its paired mate, bringing parity of expression between males and females (54).
The phenomenon that the X and Y originated from homologous autosomes and that X-linked genes in one mammal would be shared by all was first suggested by Ohno (55). The same process is observed in ZZ/ZW birds and some advanced snakes, which have highly heteromorphic sex chromosomes with extensively atrophied W chromosomes. Some snakes, however, and all amphibians have homomorphic sex chromosomes that are virtually indistinguishable, and it is in many of these species that the environmentally induced interchangeability of sex-determining mechanisms is occasionally observed. No GSD species with heteromorphic sex chromosomes is known to display this phenomenon, and evolutionary drive is consistent with GSD eventually dominating TSD due to its irreversibility. Once atrophy of the sex-determining chromosome has commenced, reversal is unlikely.
EVOLUTIONARY ACCUMULATION OF TESTIS-SPECIFIC GENES TO THE Y
Along with the decay of most of the ancestral autosomal genes on the heterogametic sex chromosome controlling GSD (the Y chromosome in mammals), there is a parallel accumulation of genes on the Y that control spermatogenesis. This inevitably occurs because the region next to the testis determining gene, which does not recombine during meiosis, is a “safe harbor” for genes that are beneficial to the male but detrimental to the female. Sexually antagonistic male-benefit genes that are testis-specific and therefore enhance male fertility have thus been accumulated and amplified on the nonrecombining region of the Y over the course of 300 million years by three different molecular evolutionary processes: transposition, retroposition, and persistence (56–59). Thus, a functionally coherent concentration of testis-specific genes has arisen on a labile Y chromosome that is subject to deletions and inversions caused by massive direct and inverted regions of nucleotide identity (amplicons and palindromes) (60, 61) and is a significant cause of human male infertility (62, 63). There is, however, a fragile balance between gene conversion and repair by the palindromes to maintain these genes and their frequent deletion due to illegitimate homologous recombination between massive ampliconic repeat sequences (64–66).
A very similar driving process is likely to be occurring on the W chromosome of birds and snakes, albeit with different arrangements of genes and repetitive sequences (67). Despite the risk to fertility, the recurring, independent emergence of sex determination based on genes located on sex chromosomes is a foolproof mechanism for ensuring a balanced ratio of males to females in subsequent generations. The potential reduction in spermatogenesis due to deletions of the atrophying Y chromosome (in the absence of sperm competition) is well balanced by the assurance of a stable sex-ratio, protected from environmental vicissitudes (68).
MOLECULAR RELATIONSHIPS BETWEEN TSD AND GSD
Temperature-dependent sex determination achieves a balanced sex ratio through two principle formats. In one format, temperatures above or below an intermediate range lead to all-male or all-female clutches, respectively (depending on the species). In the other format, intermediate temperatures give rise to one sex and temperatures above and below the intermediate range give rise to the opposite sex (14, 28). All crocodilians studied to date use the latter format. The temperature-sensitive switch determining sex remains elusive but operates at one particular developmental or temperature-sensitive period during egg incubation, when the sexual identity is fixed. In the alligator, for example, just a few days in the middle third period of egg development is temperature sensitive.
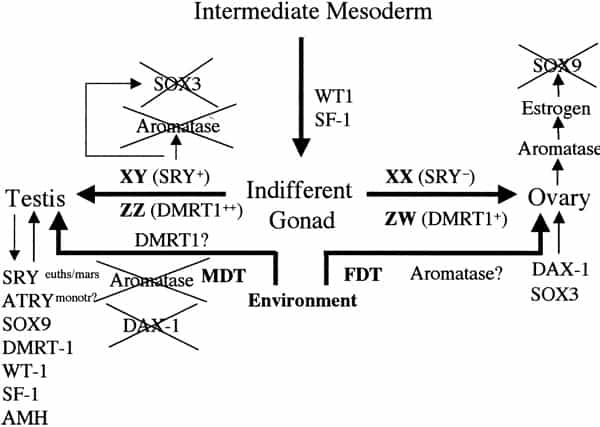
Sex determination and differentiation pathways in GSD and TSD animals. Sex determination pathways and the genes involved or suspected of being involved are represented by thick arrows. Thin arrows represent gene pathways involved in sexual differentiation. In eutherian mammals with male heterogamety (XX females and XY males), SRY is the male-determining switch. The equivalent switch in bird and snake female heterogamety (ZZ males and ZW females) is not known, although the testis-specific gene DMRT1 is absent on the W and hence only one copy is present in females, which suggests a possible chromosome-dependent, dose (+female or ++male) response mechanism of sex determination. SRY is also implicated in downregulation of SOX3 and aromatase expression and upregulation of SOX9. In TSD, male-determining temperatures (MDT) are permissive for SOX9, DMRT1, and AMH expression and refractory to DAX1 and SOX3 expression, both involved in female sexual differentiation. Unlike SRY, none of these genes are expressed as a prelude to sex determination (as is also the case for DMRT1 in birds), hence the controlling switch through which temperature operates in TSD remains elusive. The dosage of DMRT1 expressed at male- or female-permissive temperatures might be significant in this respect. Orthologues of these genes (except SRY) are commonly expressed during sexual differentiation across mammalian and reptilian species, regardless of sex-determining mechanism. Miller. Sex determination and dinosaur extinction. Fertil Steril 2004.
Hence, depending on the species and its pivotal temperature, reptile embryos that use TSD develop into either males or females, and clutches of all-male or all-female offspring can easily be obtained under laboratory conditions (28). In the wild, close-to-normal sex ratios are often observed as nest temperatures fluctuate around these transitional or pivotal “optima,” facilitating the development of both sexes and occasionally intersex offspring. Single-sex clutches, however, are also frequently observed, with the sex ratio depending on the ratio of all-male to all-female clutches in a given area (69). Significantly, TSD has also been reported in a viviparous lizard, in which nest temperatures are of no relevance, which suggests that it might also operate among certain live-birth reptiles (70).
The Z/W sex chromosomes of birds are similar to reptiles that use GSD (notably snakes), as well as some amphibians. Furthermore, most of the genes in the mammalian sex-determining cascade have orthologues in both GSD and TSD species, including DMRT1, WT1, SF1, SOX9, AMH, and DAX1 I (Table 1 and Fig. 2) (71). In the American alligator modelAlligator mississippiensis, the orthologue of the hu- man autosomal gene SOX9 is of particular interest because of its essential role in testis development in both TSD and GSD systems. Ectopic expression of the gene in transfected XX mice causes female-to-male sex reversal, which shows that SOX9 can effectively substitute for SRY (72). Moreover, mutations in SOX9 can result in human XY male-to-female sex reversal (73, 74) due to loss or inactivation of one its alleles on chromosome 17.
In the alligator and in mice, SOX9 is initially expressed in the genital ridge of both males and females before being upregulated in and specific to the developing testis (hence only at male-permissive temperatures in TSD). Concomitant downregulation is observed in females. Being autosomal in mammals, SOX9 is likely to be expressed biallelically, which suggests that one copy is incompatible with male development even in the presence of SRY. However, SOX9 is unlikely to be the male-determining switch in TSD because it is upregulated after commitment to male development has occurred (75). For a more detailed account of genes involved in sex determination, including additional gene orthologues of TSD reptiles, the reader is referred to the excellent reviews of Marshall Graves and Shetty (7) and Western and Sinclair (71).
In GSD species, sex determination is set before differentiation of the male or female characteristics commence. Although specific sex-determining genes are involved, we suspect from both de novo and experimentally generated sex-reversal cases that gene dosage is also an important factor. In this respect, differential egg incubation temperatures might affect gene dosage of a critical gene or genes that together commit the TSD embryo to one sex or the other. Only detailed quantitative studies of transcript or protein levels in developing embryos will elucidate this, and the recent development of RNA interference technology offers another potentially rewarding route toward testing this idea (see Conclusions). In support of the notion that differential gene dosage rather than particular genes operate to determine sex in TSD species, subsequent gonadal development is much more sensitive to hormonally induced sex reversals than is the case in mammals.
AROMATASE AND SEX DETERMINATION
For both GSD and TSD, the activity of aromatase is pivotal in the conversion of T to E2, thus aromatase is a major contributor to the development of female characteristics, particularly secondary sexual characteristics in mammals. Aromatase is known to be a key regulatory enzyme in the sexual development of all TSD animals studied to date (28). Hence, at permissive (for female development) temperatures, aromatase levels rise during early embryogenesis accompanied by rises in E2 and ovarian development. At nonpermissive temperatures, aromatase levels remain low while T rises accompanied by testis development. It is a simple matter to provide exogenous estrogens to developing eggs incubating at male-determining temperatures and force them to hatch as females. The reverse is not as readily achieved with exogenous T, although inhibitors of aromatase can masculinize developing females (76). Similar experiments on chickens show that male-to-female sex reversal is observed after intra-ova injection of E2, but birds normally revert to a male phenotype as they mature (77–79). Mammalian development is less sensitive to exogenous steroidogenic factors, although there is some controversial speculation that environmental estrogens might cause some reduction in human male fertility (80–84). However, complete chemically induced sex-switching, as extensive as that observed in TSD reptiles exposed to estrogens, has not been observed in mammals, although some birds and fish can switch sex during their lives (9, 77, 85–88).
These experiments demonstrate that the control over aromatase activity is the key to TSD, although how temperature exerts its control over this enzyme is still unknown. Recent experimental evidence suggests that aromatase itself is not the target because its expression is not concomitant with differential sex determination during the temperature-sensitive period (89, 90).
PERSISTENCE OF TSD IN REPTILES
Unlike GSD, animals making use of TSD are automatically placed under environmental constraints with regard to balancing the sex ratio. For TSD to have persisted in some animals, it must, however, offer some advantages in relation to reproductive fitness. Given that higher temperatures will usually result in faster growth rates and hence larger adult size, it follows that the sex-determining temperature will usually favor the animal best suited to exploit that environmental niche. Female turtles are generally larger than male turtles, and females are determined by higher egg incubation temperatures than males (14).
The fecundity of female turtles is clearly an important factor in their larger size. Male lizards are larger than females, however, and males are determined by higher incubation temperatures. Crocodile males are also larger than females, but in these animals, temperatures both below and above the male optima are feminizing. The choice of the male as the larger sex is probably related to the observed intermale rivalry for females. In the case of crocodilians, it has been suggested that the narrow range of temperatures required to facilitate male development reflects a wider variation in temperature flux due to the wider environmental range of these animals and their eggs’ consequent exposure to a wider variety of ambient temperatures (14).
Animals using TSD, therefore, have a convenient way of tailoring their physical and behavioral characteristics according to sex, to efficiently benefit from and fit in with their environment. The various “differential fitness” models for adaptation in TSD have developed from these notions and provide useful explanations for why egg incubation temperature should have effects that go beyond sex determination and include the adaptive significance of TSD. However, TSD does not protect animals from deleterious sex-ratio skewing under conditions of massive environmental shifts. The reader is directed to the review by Shine (69) for more detailed information on this complex subject.
MATHEMATIC MODELING OF SKEWED SEX-RATIO EFFECTS
The best fossil evidence is unable to conclusively show that the strong sexual dimorphism and hence differential fitness exhibited by crocodiles was also present in dinosaurs. Moreover, the known types of nest, nesting sites, and nesting behavior used by these animals is too incomplete to shed light on whether these factors might have played an important role in dinosaur sex determination (41). Given the warmer Jurassic climate, in which global temperatures were higher than they are today, TSD might have given animals the flexibility to exploit niches that might not have otherwise been available to them, and there is no reason to believe that dinosaurs would not also have benefited. The simplest population growth models consider growth rates (e.g., dx/dt: see equation below) to be directly proportional to the population size. However, this approach does not include the necessary breeding dynamics, which can be achieved by splitting the population into males (x) and females (y). In this case, population growth is governed by how many females (π) a male can mate with; if there are more than enough females (i.e., y – πx > 0), then the growth is dictated by the size of the male population, x; otherwise, if there are insufficient females (y – πx < 0), the population dynamics then depend on the female population size, y. Switching between the two situations is achieved mathematically by the heaviside function, H, which is zero or unity depending on whether its argument is respectively negative or positive. Consider a birth rate, δ, a male death rate, Kx, a female death rate, Ky, and a time-varying percentage of births that are male, λ(t); the population growth is then modeled by the following quasilinear dynamical system:

The function λ(t) in the above equation is the time-dependent proportion of births that are male, and it takes the following functional form:
where λ0 and λ∞ are, respectively, the initial and final proportions of births that are male, tav is the average cycle at which the transition between λ0 and λ∞ occurs, and n describes how quickly the change should take place (rate), that is the duration in cycles required to return the ratio back to normality (λ∞ ).

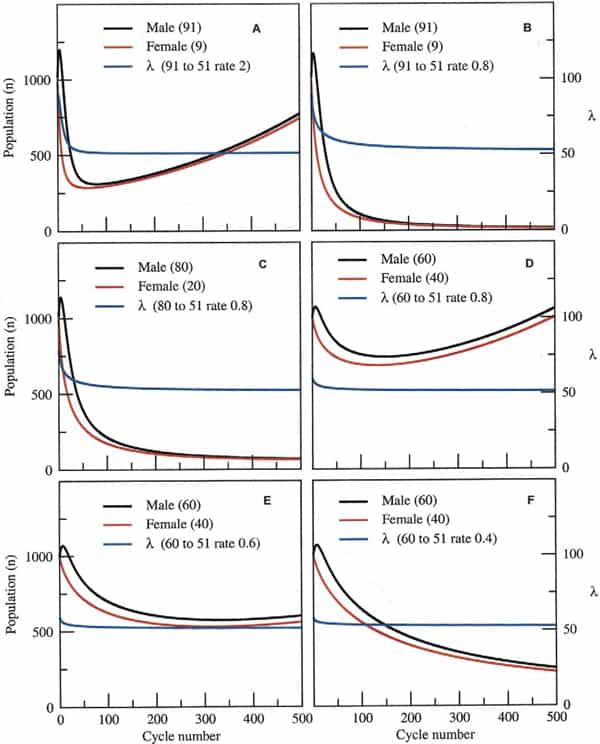
The questions therefore, are how much skew could a species endure and for how long before its extinction was inevitable? Taking a surviving population of 1000 pairs (x = y = 1000 at t = 0), at the time of the disaster, for example, and assuming a birth rate and death rate of 0.25 and 0.12, respectively, this leaves a margin of 0.01 for population growth (0.25–0.24). Assuming that the sex ratio skewed from 50:50 (male/female) to 91:9 after the disaster and that, on average, each male mated with four females in each reproductive cycle (probably a conservative estimate), populations would still robustly recover after an initial decline, provided the skew was lost within 50 subsequent cycles (Fig. 3A). However, populations would inexorably decline toward extinction if the skew was to take longer than 50 cycles to abrogate, because the animals’ rate of population growth would not be high enough to sustain them during this period (Fig. 3B). This would also be the case given a more modest skew of 80:20 and the same rate of skew abrogation (Fig. 3C). A moderate skew of 60:40 at this same rate of recovery, however, would permit population expansion after an initial decline (Fig. 3D). Finally, fine-tuning the permitted recovery period from an initial skew of 60:40 shows the fine dividing line between likely recovery of the population (Fig. 3E) or its inevitable demise (Fig. 3F).
Clearly, the likelihood of extinction depends not just on the initial skew and rate of recovery in the sex ratio, but also on the birth and death rates before and after the environmental catastrophe itself. For simplicity, our model is set for a small margin in population growth, a factor that is probably generous considering the environmental changes that these animals must have encountered at the time. The model also demonstrates that a predominance of female births over an extended period is not sufficient for population collapse if some males are available (data not shown). This is because the dynamics of population growth are dependent on fewer numbers of males and only in extreme cases, in which all-female populations arise in subsequent generations, would extinction be likely.
WHY DID SOME SURVIVE?
It is clear that avian archosaurs were in a good position to survive the K-T event, 65 MYA, because they had evolved a GSD system. But why did TSD animals like crocodilians and turtles survive? These animals live at the intersection of aquatic and terrestrial environments, in estuarine waters and river beds, which might have afforded some protection against the more extreme effects of environmental change, hence giving them more time to adapt. Alternatively, the loss of marine animals living along coastal waters might have come about by a complete collapse of the supporting ecosystem. Indeed, changes in marine temperatures are thought to be responsible for the contemporary bleaching of coral reefs and the increase in toxic red tides (91).
CONCLUSIONS
In the absence of definitive evidence, we can infer that TSD was the forerunner to GSD (Fig. 1). In reptiles, this conclusion is based not only on phylogenetic evidence but on the latest molecular genetic data. In this scenario, most or all of the genes controlling testis or ovary formation in TSD had already evolved before the appearance of GSD in reptiles. Probably all forms of GSD use molecular switches that were originally derived from one of these genes. For example, a single gene (probably related to SOX3) acquiring a stabilizing role as the primary switch for sex determination was all that was needed for GSD to first evolve in mammal like synapsid species some 250–300 MYA. Because this group is credited with being the forerunner of the mammals, these sex chromosomes might have been the forerunners of the modern mammalian XY pair that uses SRY as the sex-determining switch. The widespread expression of SRY in the tissues of some mammals supports additional developmental and regulatory roles for this gene other than in sex determination. The ZW chromosomes independently developed after the co-opting of DMRT1 into a sex-determining role in other reptile groups at around this time. There then followed an intermediate period, wherein essentially homologous XY and ZW-like homomorphic sex chromosomes persisted in these groups.
These autosomally derived chromosomes were the prototype homomorphic ZZ/ZW pairs that we still see today in boid snakes and ratite birds and the XX/XY pairs observed in monotremes. With the selective loss of meiotic recombination, the W and Y eventually degenerated due to addition and attrition and to conflict between selfish sex-determining elements, thus producing the markedly heteromorphic ZW sex chromosomes seen in advanced snakes and carnite birds and the XY chromosomes seen in eutherian mammals and most marsupials. Most lizards studied to date use largely homomorphic XY chromosomes, a property shared with monotremes (92).
The phylogenetic relationship between crocodiles and dinosaurs also supports the hypothesis that the archosaurian lineage had diverged, with TSD as their default sex-determining mechanism. The relatively clement environments enjoyed by animals in the Jurassic and early Cretaceous periods would not have driven their switching to GSD (93, 94). The net result of post–K-T climatic change, therefore, could have been the skewing of the sex ratio toward a preponderance of males for a period that was too prolonged to permit population recovery. It is unlikely that a preponderance of females, even over very long periods covering many hundreds of reproductive cycles, would have led to extinction.
Several experimental tests can be applied to existing reptiles that might shed more light on their susceptibility and adaptive responses to sex ratio skew, as well as the molecular basis of TSD.
- Based on mathematic modeling, it should be possible to expose breeding TSD populations to temperatures that lie just beyond either side of pivotal to slightly skew the sex ratio. A positive adaptive response would be one in which the sex ratio eventually returned to normal while the temperature extreme was maintained.
- The molecular control of sex determination in TSD could be investigated with combinations of quantitative reverse transcriptase polymerase chain reaction and RNA interference. These tests will allow investigators to either passively determine specific gene expression dosage de novo at male- or female-permissive temperatures or to actively downregulate specific genes at these temperatures and to monitor its effect on sex determination.
- Animals that narrowly escaped extinction should be genetically less diverse than animals that were never at the same risk (bottleneck hypothesis). It would be revealing to use available markers of genetic variability to compare the genetic variability of TSD vs. GSD species of reptiles.
- Examining the molecular relationships between the orthologous genes involved in testis development should help unravel the timing of events leading up to the appearance of GSD.
These ideas have some bearing on emerging threats brought about by human activity to existing species that use TSD. With global warming, it is quite possible that some populations of turtles will be at risk of catastrophic sex-ratio distortion in areas where local temperatures are set to rise by 2°C–5°C in the next century (35), particularly if females become scarce. Also cause for concern is the possibility that synthetic phthalates and other industrial plasticizers with xeno-estrogenic effects will synergize with rising temperatures, causing more widespread cases of sex-ratio skewing in susceptible species (95). Finally, sex-ratio skewing in human populations in which female offspring are undervalued can produce remarkably catastrophic population decline in just a few generations.
REFERENCES
- Lovell Badge R, Hacker A. The molecular genetics of Sry and its role in mammalian sex determination. Philos Trans R Soc Lond B Biol Sci 1995;350:205–14.
- Daneau I, Houde A, Ethier JF, Lussier JG, Silversides DW. Sry gene in bull and boar show greater similarity to human than to mouse gene. Biol Reprod 1994;50:157.
- Hacker A, Capel B, Goodfellow P, Lovellbadge R. Expression of Sry, the mouse sex-determining gene. Development 1995;121:1603–14.
- Graves JA. The evolution of mammalian sex chromosomes and the origin of sex determining genes. Philos Trans R Soc Lond B Biol Sci 1995;350:305–11; discussion 311–2.
- Delbridge ML, Graves JA. Mammalian Y chromosome evolution and the male-specific functions of Y chromosome-borne genes. Rev Reprod 1999;4:101–9.
- Marshall Graves JA. The rise and fall of SRY. Trends Genet 2002;18: 259 –64.
- Marshall Graves JA, Shetty S. Sex from W to Z: evolution of vertebrate sex chromosomes and sex determining genes. J Exp Zool 2001;290: 449 –62.
- Shan Z, Nanda I, Wang Y, Schmid M, Vortkamp A, Haaf T. Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds. Cytogenet Cell Genet 2000;89:252–7.
- Nanda I, Zend-Ajusch E, Shan Z, Grutzner F, Schartl M, Burt DW, et al. Conserved synteny between the chicken Z sex chromosome and human chromosome 9 includes the male regulatory gene DMRT1: a comparative (re)view on avian sex determination. Cytogenet Cell Genet 2000;89:67–78.
- Johnston CM, Barnett M, Sharpe PT. The molecular biology of temperature-dependent sex determination. Philos Trans R Soc Lond B Biol Sci 1995;350:297–303.
- Pieau C. Sex ratio in the embryos of 2 chelonians (Testudo graeca L. and Emys orbicularis L. born of artificially incubated ova). C R Acad Sci Hebd Seances Acad Sci D 1971;272:3071–4.
- Pieau C. Temperature effects on the development of genital glands in the embryos of 2 chelonians, Emys orbicularis L. and Testudo graeca L. C R Acad Sci Hebd Seances Acad Sci D 1972;274:719–22.
- Yntema CL. Effects of incubation temperature on sexual differentiation in the turtle Chelydra serpentine. J Morphol 1976;150:453–62.
- Head G, May RM, Pendleton L. Environmental determination of sex in the reptiles. Nature 1987;329:198 –9.
- Bull JJ, Moon RG, Legler JM. Male heterogamety in kinosternid turtles (genus staurotypus). Cytogenet Cell Genet 1974;13:419 –425.
- Bull JJ, Vogt RC. Temperature-dependent sex determination in turtles. Science 1979;206:1186 –8.
- Bull JJ. Sex determining mechanisms: an evolutionary perspective. Experientia 1985;41:1285–96.
- Bull JJ, Hillis DM, O’Steen S. Mammalian ZFY sequences exist in reptiles regardless of sex-determining mechanism. Science 1988;242: 567–9.
- Wibbels T, Bull JJ, Crews D. Chronology and morphology of temperature-dependent sex determination. J Exp Zool 1991;260:371–81.
- Wibbels T, Bull JJ, Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J Exp Zool1991;260:130 –4.
- Wibbels T, Bull JJ, Crews D. Steroid hormone-induced male sex determination in an amniotic vertebrate. J Exp Zool 1992;262:454–7.
- Wibbels T, Gideon P, Bull JJ, Crews D. Estrogen- and temperature-induced medullary cord regression during gonadal differentiation in a turtle. Differentiation 1993;53:149 –54.
- Crews D, Bull JJ, Wibbels T. Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol 1991;81:357–64.
- Crews D, Bergeron JM. Role of reductase and aromatase in sex determination in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. J Endocrinol 1994;143:279 – 89.
- Crews D. Temperature, steroids and sex determination. J Endocrinol 1994;142:1–8.
- Crews D, Bergeron JM, Bull JJ, Flores D, Tousignant A, Skipper JK, Wibbels T. Temperature-dependent sex determination in reptiles: proximate mechanisms, ultimate outcomes, and practical applications. Dev Genet 1994;15:297–312.
- Fergusson MW, Joanen T. Temperature of egg incubation determines sex in Alligator mississippiensis. Nature 1982;296:850 –3.
- Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci 1999;55:887–900.
- Janzen FJ, Paukstis GL. Environmental sex determination in reptiles. Nature 1988;332:790.
- Rage J-C. Latest Cretaceous extinctions and environmental sex determination in reptiles. Bull Soc Geol France 1998;169:479–83.
- Alvarez LW. Mass extinctions caused by large bolide impacts. Phys Today 1987;40:24 –33.
- Lopez-Martinez N, Ardevol L, Arribas ME, Civis J, Gonzales-Delgado A. The geological record in non-marine environments around the K/T boundary. Bull Soc Geol France 1998;169:11–20.
- Lopez-Martinez N, Canudo JI, Ardevol L, Suberbiola XP, Prue-Etxebarrai X, Cuenca-Bescos G, et al. New dinosaur sites correlated with Upper Maastrictian pelagic deposits in the Spanish Pyrenees: impliactions for the dinosaur extinction pattern in Europe. Cretaceous Res 2001;22:41–61.
- Cloudsley-Thompson J. Multiple factors in the reptile extinctions of the Cretaceous period. Biologist (London) 2001;48:177–81.
- Janzen FJ. Climate change and temperature-dependent sex determination in reptiles. Proc Natl Acad Sci U S A 1994;91:7487–90.
- Serono PC. The evolution of dinosaurs. Science 1999;284:2137–47.
- Norell M, Ji Q, Gao K, Yuan C, Zhao Y, Wang L. Palaeontology: ‘modern’ feathers on a non-avian dinosaur. Nature 2002;416:36–7.
- Norell MA, Clarke JA. Fossil that fills a critical gap in avian evolution. Nature 2001;409:181–4.
- Xu X, Norell MA, Wang XL, Makovicky PJ, Wu XC. A basal troodontid from the Early Cretaceous of China. Nature 2002;415:780–784.
- Palmer BD, Guillette LJ Jr. Alligators provide evidence for the evolution of an archosaurian mode of oviparity. Biol Reprod 1992;46:39 –47.
- Geist NR, Jones TD. Juvenile skeletal structure and the reproductive habits of dinosaurs. Science 1996;272:712–4.
- Rogers SW. Allosaurus, crocodiles, and birds: evolutionary clues from spiral computed tomography of an endocast. Anat Rec 1999;257:162– 73.
- Ruben JA, Jones TD, Geist NR, Hillenius WJ. Lung structure and ventilation in theropod dinosaurs and early birds. Science 1997;278: 1267–70.
- D’Cotta H, Fostier A, Guiguen Y, Govoroun M, Baroiller JF. Aromatase plays a key role during normal and temperature-induced sex differentiation of tilapia Oreochromis niloticus. Mol Reprod Dev 2001; 59:265–76.
- Baroiller JF, Guiguen Y. Endocrine and environmental aspects of sex differentiation in gonochoristic fish. EXS 2001;91:177–201.
- Janzen FJ, Paukstis GL. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q Rev Biol 1991;66:149 – 79.
- Scherer G, Schmid M. Genes and mechanisms in vertebrate sex determination. Introduction. Exs 2001;91:XI–XII.
- Koopman P. Sry and Sox9: mammalian testis-determining genes. Cell Mol Life Sci 1999;55:839 –56.
- Rice WR. Degeneration of a nonrecombining chromosome. Science 1994;263:230 –2.
- Rice WR. Sexually antagonistic male adaptation triggered by experi- mental arrest of female evolution. Nature 1996;381:232–4.
- Dournon C, Houillon C, Pieau C. Temperature sex-reversal in amphibians and reptiles. Int J Dev Biol 1990;34:81–92.
- Toder R, Wakefield MJ, Graves JA. The minimal mammalian Y chromosome—the marsupial Y as a model system. Cytogenet Cell Genet 2000;91:285–92.
- Just W, Rau W, Vogel W, Akhverdian M, Fredga K, Graves JA, Lyapunova E. Absence of Sry in species of the voleEllobius. Nat Genet 1995;11:117–8.
- Jegalian K, Page DC. A proposed path by which genes common to mammalian X and Y chromosomes evolve to become X inactivated. Nature 1998;394:776 –80.
- Ohno S. Sex chromosomes and sex-linked genes. Berlin: Springer-Verlag, 1967.
- Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science 1997;278:675–9.
- Lahn BT, Page DC. Retroposition of autosomal mRNA yielded testis-specific gene family on human Y chromosome. Nat Genet 1999;21: 429 –33.
- Lahn BT, Page DC. Four evolutionary strata on the human X chromosome. Science 1999;286:964 –7.
- Saxena R, Brown LG, Hawkins T, Alagappan RK, Skaletsky H, Reeve MP, et al. The DAZ gene cluster on the human Y chromosome arose from an autosomal gene that was transposed, repeatedly amplified and pruned. Nat Genet 1996;14:292–9.
- Kuroda-Kawaguchi T, Skaletsky H, Brown LG, Minx PJ, Cordum HS, Waterston RH, et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet 2001;29:279 –86.
- Repping S, Skaletsky H, Lange J, Silber S, Van Der Veen F, Oates RD, et al. Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet 2002;71:906 –22.
- Silber SJ, Alagappan R, Brown LG, Page DC. Y chromosome deletions in azoospermic and severely oligozoospermic men undergoing intracy- toplasmic sperm injection after testicular sperm extraction. Hum Reprod 1998;13:3332–7.
- Silber SJ, Repping S. Transmission of male infertility to future generations: lessons from the Y chromosome. Hum Reprod Update 2002;8: 217–29.
- Silber S. The disappearing male. In: Jansen R, Mortimer D, eds. Towards reproductive certainty—fertility and genetics beyond 1999. New York, London: The Parthenon Publishing Group, 1999:499–505.
- Rozen S, Skaletsky H, Marszalek JD, Minx PJ, Cordum HS, Waterston RH, et al. Abundant gene conversion between arms of palindromes in human and ape Y chromosomes. Nature 2003;423:873–6.
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003;423:825–37.
- Shetty S, Griffin DK, Graves JA. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res 1999;7:289–95.
- Short RV. The evolution of human reproduction. Proc R Soc Lond B Biol Sci 1976;195:3–24.
- Shine R. Why is sex determined by nest temperature in may reptiles? Trends Ecol Evol 1999;14:186–9.
- Robert KA, Thompson MB. Sex determination. Viviparous lizard selects sex of embryos. Nature 2001;412:698–9.
- Western PS, Sinclair AH. Sex, genes, and heat: triggers of diversity. J Exp Zool 2001;290:624–31.
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet 2001;28:216–7.
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 1994;372:525–30.
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 1994;79:1111– 20.
- WesternPS,HarryJL,GravesJA,SinclairAH.Temperature-dependent sex determination in the American alligator: AMHprecedes SOX9 expression. Dev Dyn 1999;216:411–9.
- Richard-Mercier N, Dorizzi M, Desvages G, Girondot M, Pieau C. Endocrine sex reversal of gonads by the aromatase inhibitor Letrozole (CGS 20267) in Emys orbicularis, a turtle with temperature-dependent sex determination. Gen Comp Endocrinol 1995;100:314–26.
- Vaillant S, Dorizzi M, Pieau C, Richard-Mercier N. Sex reversal and aromatase in chicken. J Exp Zool 2001;290:727–40.
- Rashedi PM, Maraud R. Secretion of the anti-mullerian hormone by the gonads of experimentally sex reversed female chick embryos. Gen Comp Endocrinol 1987;65:87–91.
- Bruggeman V, Van As P, Decuypere E. Developmental endocrinology of the reproductive axis in the chicken embryo. Comp Biochem Physiol A Mol Integr Physiol 2002;131:839 –46.
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Br Med J 1992;305: 609 –13.
- Olsen J. Is human fecundity declining—and does occupational exposures play a role in such a decline if it exists? Scand J Work Environ Health 1994;20:72–7.
- Giwercman A, Bonde JP. Declining male fertility and environmental factors. Endocrinol Metab Clin North Am 1998;27:807–30, viii.
- Vanderschueren D. Is male fertility declining? Verh K Acad Geneeskd Belg 1999;61:433–40.
- Jouannet P, Wang C, Eustache F, Kold-Jensen T, Auger J. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS 2001;109:333–44.
- Allen E, Cooper JE. Sex reversal in birds. Vet Rec 1997;140:636.
- Clinton M. Sex determination and gonadal development: a bird’s eye view. J Exp Zool 1998;281:457–65.
- Gray LE Jr. Xenoendocrine disrupters: laboratory studies on male reproductive effects. Toxicol Lett 1998;102–3; 331–5.
- St Mary CM. Sex allocation in a simultaneous hermaphrodite, the blue banded Goby (Lythypnus dalli). Behav Ecol 1994;5:304–12.
- Gabriel WN, Blumberg B, Sutton S, Place AR, Lance VA. Alligator aromatase cDNA sequence and its expression in embryos at male and female incubation temperatures. J Exp Zool 2001;290:439–48.
- Belaid B, Richard-Mercier N, Pieau C, Dorizzi M. Sex reversal and aromatase in the European pond turtle: treatment with letrozole after the thermosensitive period for sex determination. J Exp Zool 2001;290: 490 –7.
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, et al. Emerging marine diseases—climate links and anthropogenic factors. Science 1999;285:1505–10.
- Archie JW, Cole CJ, Vilella OF. A review of phylogenetic hypotheses for lizards: implications for ecological and evolutionary studies. Bull Am Museum Natural History 1992:1–110.
- Reis PM, Zeigler AM, Valdes PJ. Jurassic phytogeography and climate: new data and model comparisons. In: Huber BT, McLeod KG, Wing SL, eds. Warm climates in earth history. Cambridge, United Kingdom: Cambridge University Press, 2000.
- Deconto RM, Brady EC, Bergengren J, Hay WW. Late Cretaceous climate, vegetation and ocean interactions. In: Huber BT, McLeod KG, Wing SL, eds. Warm climates in earth history. Cambridge, United Kingdom: Cambridge University Press, 2000:275–97.
- Crain DA, Guillette LJ Jr. Reptiles as models of contaminant-induced endocrine disruption. Anim Reprod Sci 1998;53:77–86.

