
A Fertility First: Human Egg Cells Grow Up in Lab
By Hadley Leggett
Wired.com, July 14, 2009
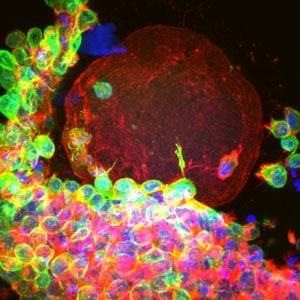
For the first time, scientists have managed to grow mature human eggs from immature cells in the lab, a technique that may eventually help save the fertility of female cancer patients who aren’t eligible for traditional egg harvest.
Researchers from Northwestern University took immature egg cells, encased in a protective sac called a follicle [see video], from 14 women who wanted to preserve their fertility before undergoing chemotherapy. By placing the cells in a unique three-dimensional growing environment for 30 days, the scientists coaxed the cells into becoming what appear to be healthy, functional human eggs.
“It is a major first,” said infertility expert, and colleague, Sherman Silber of St. Luke’s Hospital in St. Louis. “No one has yet tested the eggs by in-vitro fertilization and pregnancy, but they look quite normal and we are all excited about it.”
The traditional way to preserve a female cancer patient’s fertility is to surgically remove fully mature eggs from her ovary, fertilize the eggs immediately in the lab, and freeze the resulting embryos. But since only one follicle matures each month for ovulation, that method requires two to six weeks of hormone therapy to generate enough mature eggs for harvest.
The cancer patient usually does not have this much time to waste,” Silber said. “And to give her any kind of assurance, you would have to put her through three to six or more such cycles to get enough eggs to be comfortable. With ovary tissue freezing, we will get hundreds of thousands of eggs, and the patient can get a much greater measure of security for her future fertility.”
In addition, giving high doses of hormones is dangerous for patients with certain types of cancer, such as breast or ovarian tumors, and the traditional method won’t work for girls who haven’t gone through puberty. If physicians could take immature ovarian follicles and grow them into eggs outside the body, they could skip the hormone step altogether.
Until now, however, no one has been able to grow human ovarian follicles in the lab. Most previous attempts had involved trying to culture the cells in a two-dimensional environment, but it turns out that any kind of pressure on the egg cells inhibits their growth.
“Scientists had been putting them on a flat piece of plastic for years,” said fertility researcher Teresa Woodruff of Northwestern University, a co-author on the paper published Monday in Human Reproduction. “When you do that, the cells around the egg begin to move away, and the connection between the egg and its supporting cells is lost.”
The supporting cells are critical, Woodruff said, because they provide the hormones and nutrients that the egg needs to grow. To create the ideal growing environment for a follicle, the researchers collaborated with biomedical engineers who specialize in biomaterials.
“Our breakthrough was to use a hydrogel called alginate, which doesn’t touch or contact the follicle cells, but just supports them,” Woodruff said. It turns out that the rigidity of the gel is crucial to follicle function: If the gel is too stiff, the follicles start looking sick and making the wrong kind of hormones. “We kind of lucked out in the very beginning in that we used a very soft gel,” she said.
After incubating the follicles for 30 days in the three-dimensional matrix, the researchers discovered that they had grown to the size of mature eggs and were producing all the right hormones, such as estrogen and progesterone, in the right quantities.
The true test of the lab-grown eggs will be to see if they can undergo a final stage of cell division to be ready for fertilization.
“We did it in a mouse and got it all the way to fertilization and live birth,” Woodruff said. But regulations from the National Institutes of Health won’t let scientists fertilize human eggs for research — the final step of the process must be done by physicians in an infertility clinic for a patient who’s ready to have a baby.
“The proof would be if they could fertilize the eggs in vitro and get babies out of it,” Silber said. “But that’s an extra stage, and it’s amazing they’ve got this far.”
“Ten years ago it was considered such an impossible task that we didn’t think it would happen for another 50 years,” he said. “The amazing thing is that it turned out to be much simpler than we ever dreamed.”
If you have any questions, you may call us at (314) 576-1400.
