Sherman Silber
Infertility Center of St. Louis, St. Luke’s Hospital, St. Louis, Missouri
Academic Medical Center (AMC), Amsterdam, The Netherlands
Middle East Fertility Society Journal, January 18, 2011
Download PDF version of this article
Study of the molecular genetics of human male infertility and the Y chromosome has helped to elucidate the evolution of our X and Y chromosomes. Particularly, the study of the Y chromosome in male infertility has also helped to clarify, in a surprising and unexpected way, a likely mechanism for dinosaur extinction, the biggest question all of us have entertained from our earliest childhood days.
There have been many claims in the popular press of ”discoveries” on how the dinosaurs went extinct. These claims all relate to climate change events that occurred 65 million years ago that no one disputes occurred. But none have explored the biology of how so many animals escaped extinction while the dinosaurs and at least half of all other species did not. For example, why did large dinosaurs, as well as small dinosaurs the same size as chickens go extinct, but birds survived? Possibly the evolution of sex chromosomes holds the answer to this question.
Our studies of the Y chromosome and male infertility suggest that the default mechanism for determining the sex of offspring is the temperature of egg incubation, and that genetic sex determination (based on sex chromosomes like X and Y) has evolved many times over and over again in different ways, in different genera, as a more foolproof method than temperature variation of assuring a balanced sex ratio in offspring. The absence of such a genetic sex determining mechanism in dinosaurs may have led to a skewed sex ratio when global temperature dramatically changed 65,000,000 years ago, resulting in a preponderance of males, and consequentially a rapid decline in population.
Introduction
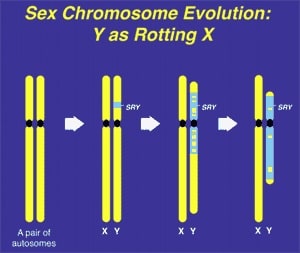
Genetically based sex determination mechanisms have evolved independently in many different genera numerous times over throughout the history of life on earth to ensure a balanced male–female sex ratio (1–13). Mammals, birds, all snakes and most lizards, amphibians, flying insects, worms, and some fish, employ specific ”sex-determining” chromosomes or genes to determine the sex of the embryo. This mechanism is generically termed GSD, for ”genetic sex determination.” In mammals, GSD is controlled by the SRY gene on the smaller Y chromosome of the male. Contrastingly, in birds and snakes, who also use GSD, the smaller sex chromosome results in female rather than male offspring.
Not all animals employ GSD for sex determination. Crocodiles and alligators, turtles, some lizards, and many fish employ environmental, or temperature-dependent sex determination (TSD) of offspring. This means that there is no genetic predetermination of gender. In these animals, it is merely the temperature at which the early embryos are incubated that determines whether the primitive gonad will differentiate into a testis or an ovary.
Temperature dependent sex determination (TSD) is thought to be the primordial mechanism that triggers either testicular or ovarian development in the early embryo (7–9). The embryonic development of the testis or the ovary from a primitive gonad is the product of a multitude of well-conserved genes. The sex-determining gene (like SRY in the mammals) is simply a trigger to activate that downstream cascade of many other genes elsewhere in the genome that actually orchestrate testis or ovarian development (Fig. 1). The various modes of genetic sex determination (GSD) have evolved many times to override TSD, completely independently and differently, in the history of life on earth (3,4,12,13). There is no similarity of the Y chromosome or the sex-determining ”trigger” gene in mammals, lizards, drosophila, or worms, except that the male determining chromosome (by definition the Y) is smaller than its counterpart (the X). In snakes and birds, the female determining trigger gene (the W by definition) is smaller than its counterpart (the Z), but they are completely different from each other in all genera.
Evolution, therefore, appears to favor the eventual repeated appearance of genetic based sex determination (GSD), we postulate, because animals not using this mechanism are at risk of extinction due to a skewed sex ratio that could result from massive global environmental temperature change. If there are multiple generations of a preponderance of male offspring, the species cannot survive. This recurring hazard favors the repeated evolution of a sex determining ”trigger” gene in all genera (to protect against the disastrous effect of environmental vicissitudes on the sex ratio of offspring).
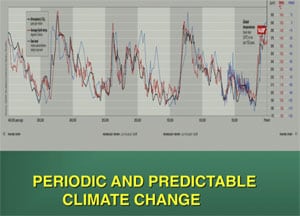
Global temperature spikes predictably occur every 100,000 years, and to a lesser extent in harmonics of every 25,000 years (1) (Fig. 2). A massive global temperature change can skew the sex ratio of TSD offspring and we have postulated that this played a role in the demise of the dinosaurs. Global warming today, which far exceeds the usual 100,000-year spike, similarly represents a current risk for extant TSD species. This theory originally suggested in 1989, was based on paleontologic evidence, before we understood the unusual sequence of the Y chromosome and the evolution of sex chromosomes (2). But now our better understanding of the DNA sequence of sex chromosomes, and of the survival of avian dinosaurs, gives even stronger credence to such a view.
Despite the survival advantage of genetic sex determination, there is, however, a survival disadvantage. The disadvantage of an evolving sex determining chromosome (which guarantees a balanced sex ratio) is its eventual decay caused by the absence of meiotic recombination during gametogenesis, and in humans the subsequent loss of spermatogenesis genes, causing male infertility. This mechanism has been confirmed in studies of infertile males, in association with the full sequencing of the Y chromosome (3,4). Hence, despite the protection that GSD affords against catastrophic global temperature shifts, and its necessity for the evolution of endothermic mammals, male infertility is increased because of the accumulation on the Y chromosome of a dense concentration of spermatogenesis genes in a region that is chromosomally unstable because of failure of chromosomal recombination during meiosis (Fig. 3). Similarly a ZZ–ZW system may have protected birds from the global K-T extinction of their cousins, the dinosaurs and flying pterosaurs. However, female birds only have one working ovary, and if it is removed, the remaining vestigial gonad becomes a testis. So we might speculate that the ZZ– ZW female bird may have avoided global extinction by employing a GSD based mechanism for sex determination, but may have paid a price for this by having only one ovary. The human male’s price for this GSD security is the risk of declining sperm count caused by Y chromosomal deletions. Thus, unexpectedly, through our efforts to understand the molecular genetics of male infertility, we may have stumbled upon one explanation for the deepest mystery of our childhood, and our biggest fear, extinction (Fig. 4).
Sex determination and extinction

Genetic modes of sex determination (GSD) in unrelated animals have arisen independently many times over (14–16). Despite highly varied ”triggering” mechanisms in a variety of vertebrates, most animals share the same or similar, highly conserved downstream genes that control sex differentiation. The genes that cause the primitive embryonic gonad to become a testis or an ovary are virtually the same for all animals on the planet. Thus, the downstream genetic programs involved in both GSD and environmental modes of sex determination (TSD) are closely related. This is perhaps not unexpected, given that the development of the testis and ovary and their constituent cells is broadly similar in amphibians, reptiles, mammals, and birds. However, the mechanisms, whether GSD or TSD, which trigger those downstream genes to direct testis or ovary development from the primordial indifferent gonads are quite different. TSD animals rely on a potentially precarious relationship with their environment where a balanced sex ratio is dependent on the temperature of egg incubation (Fig. 5) (5,17–19). Whether a sex-determining gene or random variation in temperature of egg incubation, both trigger mechanisms have the same common goal of assuring a balanced male–female sex ratio.

While the precise environmental effects of the great impact event of 65 million years ago are open to debate, it is not contested that profound global environmental changes occurred that led to the extinction of dinosaurs (20). Yet this global ”K-T” event 65 million years ago, when the dinosaurs went extinct, did not cause the mass extinction of mammals, birds, fruit flies, frogs, or snakes (Fig. 6). We suggest the reason for this could be that changes in global temperature would have had a more pronounced extinction effect on TSD-dependent animals than on animals using GSD (2). However, we must answer why alligators, crocodiles, and turtles (which are also TSD animals) survived the K-T event even though dinosaurs and pterodactyls did not, and how this theory might be tested.
Every few months another paper makes media news claiming that the cause for the extinction of the dinosaurs has been discovered. But it is always about the giant asteroid or the volcanic activity 65 million years ago, and never about the actual physiology of the animals themselves, that might have made some prone to extinction (like the dinosaurs) and others not. It is possible that without the recurrent evolution of GSD systems for sex determination, many animals, obviously not all, would go extinct because of a skewed sex ratio in their offspring.
These conclusions are based on a two-way comparison of dinosaur fossils with extant species, and construction of a mathematical population prediction model based on sex ratio skewing (21). We also evaluated the evolution of sex chromosomes by studying the mapping and sequencing of the human X and Y in fertile and infertile men (3,4,14,22–25). We postulate that the absence of genetic sex determination is very likely what could have caused the selective extinction of the dinosaurs 65 million years ago.
Relationship between dinosaurs and their relatives
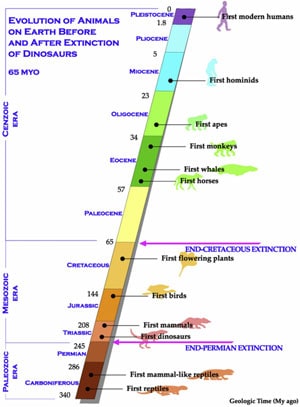
Dinosaurs and crocodiles are members of the Archosauria, a major group of diaspsids that appeared in the Early Triassic, some 245 million years ago. By the Late Triassic (208 million years ago) the dominant representatives were dinosaurs, champosaurs, pterosaurs, and crocodilians (26). Modern birds were derived from avian archosaurs that first appeared during the Jurassic (about 170 million years ago) and expanded their range in the Cretaceous period where they would have shared the skies with the dominant pterosaurs (pterodactyls). Crocodilians (TSD-dependent) and birds (GSD-dependent) are the only Archosaurian taxa that have persisted to this day (27,28). We propose that avians (birds), which are considered surviving dinosaurs, survived because of their GSD system for gender determination. Crocodilians and other TSD species (but not dinosaurs) survived because they could adapt successfully to the changing environment, or possibly their TSD system resulted in a female dominated skewing rather than males.
Studies on oviparity in the American alligator demonstrate a close physiological link between crocodilian and ancient archosaurian reproductive function (29,30). These comparisons are significant because they suggest physiological similarities between crocodilians and extinct dinosaurs. Birds developed GSD in parallel with their endothermy some 170 million years ago, whereas dinosaurs more likely employed TSD for sex determination. As GSD modes of sex determination are immune to the environmental vicissitudes that challenge animals using TSD, a strong selection bias favors the adoption of GSD in environments where a deleterious change in temperature becomes a species-threatening issue.
Studies on oviparity in the American alligator demonstrate a close physiological link between crocodilian and ancient archosaurian reproductive function (29,30). These comparisons are significant because they suggest physiological similarities between crocodilians and extinct dinosaurs. Birds developed GSD in parallel with their endothermy some 170 million years ago, whereas dinosaurs more likely employed TSD for sex determination. As GSD modes of sex determination are immune to the environmental vicissitudes that challenge animals using TSD, a strong selection bias favors the adoption of GSD in environments where a deleterious change in temperature becomes a species-threatening issue.
Molecular evolution of sex determining chromosomes
The specific chromosomes and genes responsible for GSD have evolved independently many times over and over again, in insects, worms, amphibians, reptiles, birds, and mammals indicating significant evolutionary advantages in its development. Mammals employ a XX–XY, male heterogametic system that evolved from what were originally autosomes, centered around the emergence of the SRY (testis-determining) gene on the ancestral Y chromosome between 200 and 300 million years ago (31–35). Much earlier than this, insect species developed a completely different XX–XY male heterogametic system that has no relation to the mammalian. Birds employed a female heterogametic (ZZ–ZW) system about 150 million years later. This simply means that unlike a XY system where the short sex chromosome (the Y) determines that the primitive gonad will become a testicle, the short sex chromosome (the W) of the bird determines that the primitive gonad will become an ovary. (It is a little more complicated in ZZ–ZW systems in that the double dosage of the ZZ in birds could possibly be just as sig- nificant as the single short W.)
The inherent stability of GSD in mammals and birds ironically is due to the subsequent atrophy of the sex-determining chromosome, e.g. the human Y. The driving force for this atrophy process is selective failure of meiotic recombination between the sex chromosomes, leading over time, to the gradual degradation of the non-recombining portion of the sex-determining chromosome (Fig. 1). This decay of what was previously an ordinary paired autosome has resulted in selective hyper-expression and inactivation of its paired mate (X-inactivation), bringing parity of gene dosage expression between males and females (16).
ZZ–ZW birds and some advanced snakes have highly heteromorphic sex chromosomes with extensively atrophied W chromosomes like the atrophied Y in mammals and in fruit flies. However, many snakes, and all amphibians, have homomorphic sex chromosomes, which means that they are virtually indistinguishable in size from each other. In many of these species with GSD sex chromosomes that are homomorphic, an environmentally induced interchangeability of sex determining mechanisms is occasionally observed. That is, temperature can still override the genetic sex determination system. No GSD species with heteromorphic sex chromosomes, however, is known to display this phenomenon, and evolutionary drive is consistent with GSD eventually dominating TSD due to its irreversibility once the sex-determining chromosome has atrophied. Once atrophy of the sex-determining chromosome has commenced with compensatory hyper-expression of its mate to provide gene dosage parity, there is no going back to TSD (17,36).
Evolutionary accumulation of testis specific genes to the Y and the subsequent threat to male fertility
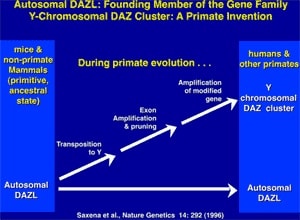
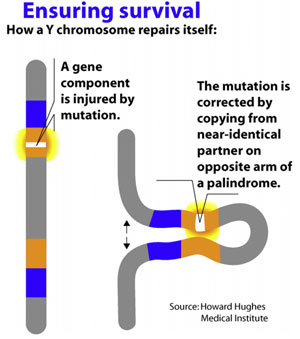
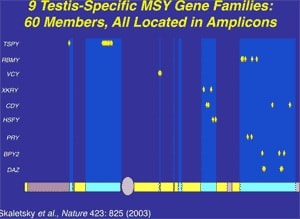
Along with the decay of most of the ancestral autosomal genes on the heterogametic sex chromosome controlling GSD (the Y chromosome in mammals), there is a parallel accumulation of genes on the Y that control spermatogenesis (14,15). This inevitably occurs because the region next to the testis-determining gene, which does not recombine during meiosis, is a ”safe harbor” for genes that are beneficial to the male but detrimental to the female (Fig. 7). ”Sexually antagonistic” male benefit genes, which are testis-specific and, therefore, enhance male fertility, but would be detrimental to female fertility, have thus been accumulated and amplified with generation of multiple copies and repeat sequences on the non-recombining region of the Y over the course of 300 million years (Fig. 8). Thus, a functionally coherent concentration of testis-specific genes in multiple copies has arisen on a labile Y chromosome that is subject to deletions and inversions caused by massive direct and inverted regions of nucleotide identity (amplicons and pal- indromes), and is a significant cause of human male infertility (3,4,23–25). There is a fragile balance between ”gene conversion” with chromosome repair by recombination between the palindrome arms, and their frequent deletion due to a mistaken homologous recombination between massive ampliconic repeat sequences on the Y (22). Thus, the inevitable evolution over and over again of sex-determining chromosomes must be very important for species survival, since it also can result in a major threat to male fertility in animals with a Y chromosome. TSD might, however, offer an advantage to some species if the environmental temperature change were to lead to more females than males. This could have been what saved some TSD species like the turtle and the crocodilians (37).
Aromatase and sex determination
For both GSD and TSD, the activity of aromatase is pivotal in the conversion of testosterone to estradiol. Aromatase is known to be a key regulatory enzyme directing ovarian development of all TSD animals studied to date(38). Hence, at permissive (for female development) temperatures, aromatase levels rise during early embryogenesis accompanied by rises in estradiol and ovarian development. At non-permissive temperatures, aromatase levels remain low while testosterone rises, accompanied by testis development. It is a simple matter to provide exogenous estrogens to developing eggs incubating at male determining temperatures and force them to hatch as females (9,10,39,40). Similar experiments on chickens show that male to female sex reversal is observed following intra-ova injection of estradiol, but birds normally revert to a male phenotype as they mature. Mammalian development is less sensitive to exogenous steroidgenic factors. However, gender switching can be observed in all TSD reptiles exposed to estrogens. These experiments demonstrate that the control over aromatase activity is the key to TSD although how temperature exerts its control over this enzyme is still unknown.
It is, therefore, likely that TSD is the ancient default mechanism for sex determination, and it simply relates to differential enzyme activity at differing incubation temperatures. The more specialized GSD (or genetic sex-determining) genes like X and Y or Z and W evolved over and over again in different genera, superimposed over the default mechanism of TSD (37).
Mathematical modeling of skewed sex ratio and testing of theory
A major question is whether the proposed skewing of sex ratio was toward male or toward female, and how severe a skewing would be required for extinction to occur, as well as how many generations of skewed sex ratio would be required to result in extinction? To answer these questions we developed a detailed mathematical model (21,41). A skewing of 90% male would result in an initial population decline, but then a robust recovery if the sex ratio balance were restored within 50 generations. However, populations would inexorably decline to extinction if the skew would take longer than 50 generations to correct. Thus, a prolonged but modest skewing toward male predominance in the gender of dinosaur offspring over 50 generations (probably only 1000 years, which is very brief in paleontologic time) would have led to extinction. A more severe male predominance could lead to a rapid extinction in just a few generations. Even a more modest skew of 80:20 would result in extinction if the skewing were to continue.
However, a skewing of 60:40 males to females would not lead to extinction even over a prolonged period. A predominance of female births even over an extended period is not sufficient for population collapse if some males are available. Therefore, it is very unlikely that a skewing toward predominantly females would result in extinction. Indeed, this may be one reason why crocodilians and turtles survived the K-T event even though dinosaurs went extinct. A preponderance of females would not be as deleterious as a preponderance of males, and might have even been advantageous to crocodilians and turtles (42,43).
Conclusions
TSD was the forerunner to GSD. Most or all of the genes controlling testis or ovary formation in TSD had already evolved prior to the appearance of GSD in early mammal-like reptiles. Probably all forms of GSD use molecular switches that were originally derived from one of these primordial testis or ovary genes. For example, a single gene related to SOX3 on the ancestral X autosome became the primary switch (SRY) for sex determination in mammals, and was all that was needed for GSD to first evolve in mammal-like reptile species some 250–300 million years ago. As this group is credited with being the forerunner of the mammals, these sex chromosomes would have been the forerunners of the modern mammalian XY pair that use SRY as the sex-determining switch. The ZW chromosomes independently developed a sex-determining role in snakes and birds about 100 million years later protecting them from the population skewing which dinosaurs suffered.
The phylogenetic relationship between crocodiles and dinosaurs supports the hypothesis that the archosaurian lineage employed TSD as their default sex determining mechanism. The relatively clement environments enjoyed by animals in the Jurassic and early Cretaceous would not have driven their switching to GSD. Thus the post K-T climatic change would have resulted in skewing of the sex ratio toward a preponderance of males for a period that was too prolonged to permit population recovery. This theory has modern day implications. The current global warming trend is so extreme that it is already affecting leatherback sea turtle survival off the coast of Costa Rica for this very same reason of the birth of a skewed sex ratio in their offspring because of a rise in the beach temperature (44). Thus, we humans may be creating conditions for another massive global extinction of TSD animals, similar to what occurred 65,000,000 years ago.
REFERENCES
- Talbot D. CO2 and the “Ornery Climate Beast”. Technol Rev 2006:41.
- Paladino FV et al. Temperature-dependent sex determination in dinosaurs? Implications for population dynamics and extinction. Geol Soc Am SP 1989;238:63–70.
- Kuroda-Kawaguchi T et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet 2001;29:279–86.
- Skaletsky H et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003;423:825–37.
- Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci 1999;55:887–900.
- Yntema CL. Effects of incubation temperature on sexual differentiation in the turtle. J. Morphol 1976;150:453–62.
- Head G, May RM, Pendleton L. Environmental determination of sex in the reptiles. Nature 1987;329:198–9.
- Bull JJ. Sex determining mechanisms: an evolutionary perspective. Experientia 1985;41:1285–96.
- Wibbels T, Bull JJ, Crews D. Chronology and morphology of temperature-dependent sex determination. J Exp Zool 1991;260:371–81.
- Wibbels T et al. Estrogen and temperature induced medullary cord regression during gonadal differentiation in a turtle. Differentiation 1993;53:149–54.
- Crews D, Bergeron JM. Role of reductase and aromatase in sex determination in the red-eared slider, a turtle with temperature-dependent sex determination. J Endocrinol 1994;143:279–89.
- Crews D. Temperature, steroids and sex determination. J Endocrinol 1994;142:1–8.
- Ferguson MW, Joanen T. Temperature of egg incubation determines sex in Alligator mississippiensis. Nature 1982;296:850–3.
- Saxena R et al. The DAZ gene cluster on the human Y chromosome arose from an autosomal gene that was transposed, repeatedly amplified and pruned. Nat Genet 1996;14:292–9.
- Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science 1997;278:675–9.
- Jegalian K, Page DC. A proposed path by which genes common to mammalian X and Y chromosomes evolve to become X inactivated. Nature 1998;394:776–80.
- Janzen FJ, Paukstis GL. Environmental sex determination in reptiles. Nature 1988;332:790.
- Janzen FJ, Paukstis GL. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q Rev Biol 1991;66:149–79.
- Janzen FJ. Climate change and temperature-dependent sex determination in reptiles. Proc Natl Acad Sci USA 1994;91:7487–90.
- Schulte P et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 2010;327:1214–8.
- Miller D, Summers J, Silber S. Environmental versus genetic sex determination: sex chromosomes and dinosaur extinction? Fertil Steril 2004;81:954–64.
- Rozen S et al. Abundant gene conversion between arms of palindromes in human and ape Y chromosomes. Nature 2003;423:873–6.
- Silber SJ et al. Y chromosome deletions in azoospermic and severely oligozoospermic men undergoing intracytoplasmic sperm injection after testicular sperm extraction. Hum Reprod 1998;13:3332–7.
- Silber SJ, Repping S. Transmission of male infertility to future generations: lessons from the Y chromosome. Hum Reprod Update 2002;8:217–29.
- Silber S. The disappearing male. In: Jansen R, Mortimer D, editors. Towards reproductive certainty-fertility and genetics beyond 1999. New York and London: The Panthenon Publishing Group; 1999. p. 499–505.
- Serono PC. The evolution of dinosaurs. Science 1999;284:2137–47.
- Norell M et al. Palaeontology: ”modern” feathers on a non-avian dinosaur. Nature 2002;416:36–7.
- Norell MA, Clarke JA. Fossil that fills a critical gap in avian evolution. Nature 2001;409:181–4.
- Palmer BD, Guillette Jr LJ. Alligators provide evidence for the evolution of an archosaurian mode of oviparity. Biol Reprod 1992;46:39–47.
- Rogers SW. Allosaurus, crocodiles, and birds: evolutionary clues from spiral computed tomography of an endocast. Anat Rec 1999;257:162–73.
- Rice WR. Degeneration of a nonrecombining chromosome. Science 1994;263:230–2.
- Ohno S. Sex chromosomes and sex-linked genes. Berlin: Springer-Verlag; 1967.
- Graves JA. The evolution of mammalian sex chromosomes and the origin of sex determining genes. Philos Trans R Soc Lond B: Biol Sci 1995;350:305–12.
- Delbridge ML, Graves JA. Mammalian Y chromosome evolution and the male-specific functions of Y chromosome-borne genes. Rev Reprod 1999;4:101–9.
- Marshall Graves JA, Shelty S. Sex from W to Z: evolution of vertebrate sex chromosomes and sex determining genes. J Exp Zool 2001;290:449–62.
- Doumon C, Houillon C, Pieau C. Temperature sex-reversal in amphibians and reptiles. Int J Dev Biol 1990;34:81–92.
- Scherer G, Schmid M. Genes and mechanisms in vertebrate sex determination. Berlin: Berkhauser, Verlag; 2001.
- D’Cotta H et al. Aromatase plays a key role during normal and temperature-inducted sex differentiation of tilapia. Mol Reprod Dev 2001;59:265–76.
- Vaillant S et al. Sex reversal and aromatase in chicken. J Exp Zool 2001;290:729–40.
- Wibbels T, Bull JJ, Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J Exp Zool 1991;1260:130–4.
- Girondot M, Pieau C. Effects of sexual differences of age at maturity and survival on population sex ratio. Evol Ecol 1993;7:645–50.
- Silber S, Geisler JH, Bolortsetseg M. Unexpected resilience of species with temperature-dependent sex determination at the Cretaceous-Palaeogene boundary. Biol Lett 2010. doi:10.1098/ rsbl.2010.0882 [published online].
- Clinton M. Sex determination and gonadal development: a bird’s eye view. J Exp Zool 1998;281:457–65.Rosenthal E. Turtles are casualties of warming in Costa Rica. The New York Times 2009:A8 [November 14].

