Article has been medically reviewed and certified by Dr. Sherman J. Silber, M.D.

Does Age Effect Male Fertility?
Seminars in Reproductive Endocrinology
Volume 9, Number 3, August 1991
Sherman J. Silber, M.D.
Decreasing Fertility of the Wife in Relation to Aging
Studies from the Office of Health Research, Statistics and Technology of the US. Department of Health have made it clear that fewer than 1% of teenage girls are infertile, but more than 20% of women in their mid-30s are infertile.(1-4)
This decrease in fertility with age is not a new phenomenon that suddenly developed in the 1980s. Many studies in the past have consistently demonstrated a decrease in fertility of older couples.
The question that this age-related decline in a couple’s fertility brings up is to what extent it is the consequence of reduced female fertility, male fertility, or both.
The first well controlled study on this issue came from France in 1982. It compared the results of artificial insemination with highly fertile donor sperm in 2193 nulliparous women who had azoospermic husbands.(5)
In order to control for the possibility that the age of the male partner might affect the infertility of an older couple, this huge series employed artificial insemination with uniformly fertile donor sperm to women of various ages over a prolonged period of time.
The cumulative success rate (as well as the pregnancy per cycle) for women 25 years of age or younger was similar to that of women over 25 but under 31 years of age. However, there was a significant decrease in the pregnancy rate for women 31 to 35 years of age, and a dramatic decrease for women over the age of 35 years.
Thus, the pregnancy rate per cycle and the overall cumulative pregnancy rate clearly went down with age in women in whom the male factor was constant, (i.e., only fertile donor sperm were used).
This large French study proved the dramatic reduction in fertility of women as they pass the age of 30 years, becoming progressively more severe during their next 10 years.
They separated the influence of the age of the woman from other variables associated with age, such as decreased frequency of sex with age as well as increased age of the husband.
Their study completely ruled out those issues by using artificial insemination with frozen donor semen.
A second problem they dealt with is that in many cases where the husband is “infertile” his sperm count is simply low, but he is definitely not “sterile.”
An extremely fecund woman may thereby become pregnant easily by a man who is extremely infertile. Thus, in any group of women seeking artificial donor insemination whose husbands were merely oligospermic, there will be a significant number of such women who are more infertile than in a random population of women, and this may not be related to age.(6)
By choosing only women whose husbands were azoospermic, the French authors completely removed that bias. Thus, it was clear that female fertility declines dramatically with age and it becomes most pronounced and observable past the age of 35 years. Further evidence to this point comes from the extremely low pregnancy rates in women 40 years of age or older undergoing in vitro fertilization. 7
The next most serious question (which has now been settled) related to decrease in female fertility with age is whether the problem is caused by the age of the ovaries (and in that respect the age of the oocyte) or if it is related in some way to the actual age of the woman.
In vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) studies using eggs donated from younger women to postmenopausal women (often as much as 20 years older) have clarified this issue incontrovertibly. 8
It has been well established that pregnancy rates with GIFT and IVF are much higher in younger women in their 20s than older women in their late 30s or early 40s. However, when embryos obtained from the eggs of younger women are placed into older women, often in their late 40s or early 50s, the pregnancy rate is just as high as that obtained with IVF or GIFT in extremely young women.
In fact, one dramatic surrogate case from South Africa involved the transfer of embryos from a 25 year-old woman who had lost her uterus to her 48 year-old mother who was menopausal but still had a uterus. The mother “grandmother” got pregnant in the first cycle with triplets, all three embryos implanting, and gave birth to three healthy grandchildren. This was probably the most dramatic single anecdotal example of the fact that a woman of any age can be highly fertile if the embryos transferred to her come from oocytes of a young woman.(9)
In fact, the pregnancy rate with donated eggs is usually well over twice as high as it is with standard IVF or GIFT. The reason for this is that the donated eggs are either coming from younger, selected donors with good ovarian function or are coming from the extra oocytes available from women who stimulated extremely well and have far more eggs than they need for their own fertilization cycle.
Thus, it is the “fertility” so to speak of the ovary, or the age of the eggs, that is important in determining the age related infertility of the woman rather than any other aspect of her reproductive anatomy.(10-12)
In summary, as the couples age, their fertility wanes. This decrease in fertility is clearly caused at least by a declining ability of the aging ovary, despite continued ovulation, to produce easily fertilizable eggs that will result in a pregnancy with a normal live birth. The next issue to be tackled is to what extent the increasing age of the male partner might also negatively affect the fertility of the older couple.
Declining Sexual Function in the Aging Male
Although until recently there has been great controversy over the issue of whether fertility declines in men as they get older, there has never been any controversy about the decline of overall sexual function. 13
With each succeeding decade after the late teen years, there is a distinct decrease in the ability of all men to obtain erections easily A greater amount of stimulation is required for obtaining erections, and a longer interval is required after orgasm and ejaculation for another erection to be achieved.
Teenage boys have erections develop spontaneously at the slightest thought of a female companion nearby, whereas men in their late 40s and 50s, although quite capable of great sexual excitement, generally need foreplay to obtain an erection sufficient for sexual relations.
Furthermore, the firmness of the erection in older men, although adequate for penetration, is not as turgid as measured objectively to that of younger men. Finally, younger men in their early 20s can often engage in sexual relations within less than half an hour after having had a previous orgasm, whereas men in their 50s may have to wait hours and perhaps even an entire day before being able to engage in intercourse again.
This interval between ejaculation and the ability to obtain another erection is called the “refractory period” and this refractory period clearly increases with aging (Fig. 1).
In young men, even after ejaculation, the penis will remain firm for some time afterward, whereas in older men the erection becomes flaccid fairly promptly after ejaculation.
The force of the ejaculatory squirt in young men is often powerful and can eject the sperm some distance. The force of the squirt, propelled by powerful contraction of the bulbocavernosus muscles, is much less in older men than in younger men. Thus, in every measurable way male potency is clearly affected by age.
Numerous scientific studies have been performed to elucidate the reason for the declining sexual performance noted in men with advancing age.(14-19) There has certainly been found to be a modest decline in serum testosterone level, along with a modest increase in follicle-stimulating hormone (FSH) and luteinizing hormone (LH) associated with advanced age in men.
But these changes are not great enough to account for the significant changes in sexual function. Furthermore, although the testosterone level does decrease modestly in men as they get older, it remains within a “normal” range.
Because of the great variability of testosterone among all men and the lack of relationship of that variability of testosterone level to sexual function in men of various ages, there is no good hormonal explanation,except in rare cases, for the universal decline in sexual function in men associated with age.
It has not escaped the notice of sociologists and psychologists specializing in the treatment of sexual dysfunction that this declining physical sexual ability in men does not necessarily mean a decline in sexual enjoyment or ability to satisfy the man’s partner. In fact, quite ironically, the fact that as men reach their middle ages and later years they require foreplay for stimulation as well as intimacy (not clearly a requirement for younger men) often leads to what is described by the female partner as an improvement in the sexual performance of their mate as they get older.
This psychological irony notwithstanding, there is an incontrovertible decline in all the measurable physical aspects of sexual function in men as they get older, and there is no clear explanation other than age itself. The more controversial issue to be discussed in the next section is whether the male partner’s fertility also declines as he gets older.
Decreased Spermatogenesis and Fertility of Men Associated with Increasing Age
It is widely viewed that although the female gradually becomes less fertile with age and eventually undergoes menopause between the ages of 45 and 55 years, the male retains his fertility well into old age and does not go through an endocrinological menopause. This point of view is in general accurate.
Men at middle age do not have hot flashes and dramatic elevations of FSH and LH associated with gonadal atrophy as women do. In fact, men have been documented in the scientific literature to retain their fertility to as old an age as 94.20 It is well known that the Senior Senator in the US. Congress, Senator Strom Thurman, fathered a child at age 81 years. Similarly in animals, bulls have been known to retain their fertility to as old as 19 Years of age (quite aged for cattle).(21,22)
Abundant spermatozoa have been found in the testes of men undergoing orchiectomy at an extremely old age.(23,24) Thus, it is clear that men do not undergo a menopause similar to women, and men in general can be expected to retain their fertility well into advanced old age.
However, there are age-related declines in male fertility and spermatogenesis that have only recently been studied with great scientific care.
These new findings help elucidate some otherwise difficult to understand cases where there seems to be a deterioration in fertility of occasional men who were clearly fertile when they were young, but later developed very severe oligospermia, and could no longer get an otherwise fertile, younger wife pregnant.
The question is: even though men can have adequate sex lives into an old age, is there a decline in fertility that parallels the decline in objectively measurable aspects of sexual function that we have already discussed in the previous section.(25)
In 1984 Johnson measured daily sperm production in a group of 89 men, ages 21 to 50 years, and compared that to a group of 43 men, ages 51 to 80 years. 26
The methodology was a random selection of such testes obtained at autopsy with a morphologic method of determining daily sperm production at the time of death. As might be expected, there was a remarkably large standard deviation (more than a 50% variation of mean value in daily sperm production from one man to another), even within the same age group.
However, if one simply looked at the mean daily sperm production of men from the older group and compared that to the mean of men from the younger group, there was an average of 30% greater sperm production in younger men than in older men.
There could be no doubt on the basis of this study that sperm production declines significantly with age in the human male, even though a 30% decline in most men would not be sufficient to render them infertile if previously they had been fertile. Yet in some men who started out on the lowest edge of fertility in youth, a significant enough reduction in sperm production could have resulted in severe infertility or sterility by middle age.
The mechanism whereby age causes a decrease in sperm production in the men is important to understand, because it bears heavily on an understanding of the difference in mechanisms of sperm production in men who are fertile versus men who are infertile, and the possibilities for treatment. First, we should review in a simplified fashion the normal stages of spermatogenesis, how we quantitate it on testicle biopsy, what stages of spermatogenesis are affected by what hormones, how these stages of spermatogenesis compare to the stages of oogenesis in the female, and how oogenesis is affected by hormones.
We will see a remarkable similarity between hormonal stimulation of different stages of spermatogenesis in the male as well as oogenesis in the female during each menstrual cycle. This will help us gain an understanding of the relative refractoriness of spermatogenesis to hyperstimulation with hormones. We will then be able to see how age affects spermatogenesis, at what stage of spermatogenesis age has its negative affect, and how this whole process differs in oogenesis.
Simplified Review of Normal Spermatogenesis and Comparison to Oogenesis
There has been little clinical literature, if any at all, about the comparison of spermatogenesis in the male and oogenesis in the female. There is a dramatic similarity in the genetic preparation of the egg for fertilization (which occurs under the influence of FSH and LH during each monthly menstrual cycle), and the changes that early spermatogonia go through in the male testes during a 3-month cycle, to result in the release of mature spermatozoa.
Although the morphology and dynamics of spermatogenesis are quite complex, a simplified review at this point will help to explain the reduction in fertility found in older men as well as develop a better understanding of male factor problems in younger men and the confusing refractoriness of such male factor patients to hormonal stimulation (even in severely oligospermic men with low or normal FSH levels).
When men have low sperm counts or high sperm counts, it is not because they are making sperm at a slower or faster rate. It is simply because there are less sperm being made. Kinetically the rate of sperm production is constant in any species.
Heller and Clermont first described the histology and kinetics of spermatogenesis in the human.(27) They determined through radioactive tracer studies that the rate of spermatogenesis in humans, or in any species, is always constant, even when sperm production is dramatically reduced.
Reduced sperm production is always caused by a reduced number of sperm “on the assembly line,” but not by any reduced speed of spermatogenesis.
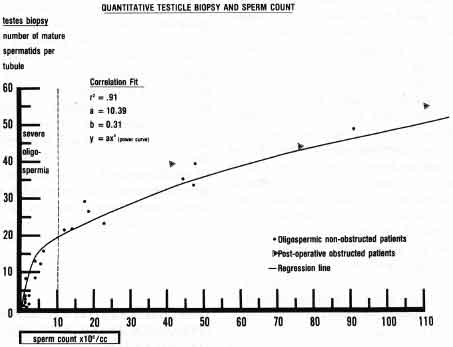
Therefore, the amount of sperm being produced by the testicle is always reflected by what is seen at any moment, either in a thin specimen of a testicle biopsy or a homogenized specimen of testicular tissue.(28-31)
Quantitation of the steps of spermatogenesis on testicle biopsy has been shown clearly to correlate with daily sperm production and sperm count in men who are not obstructed(30) (Fig. 2).
There is no correlation between sperm count and the number of sperm precursors such as spermatogonia or primary and secondary spermatocytes. In fact, most infertile men with severe oligospermia have normal size testicles, normal FSH levels, and large amounts of spermatogonia and spermatocytes, but a dramatically reduced number of spermatids, indicating a failure of the second meiotic reduction division.
Most infertile males appear to be endocrinologically quite normal but for some reason have a block at meiosis, the second reduction division stage, which in the female is equivalent to the release of the second polar body that is stimulated not hormonally, but by penetration of the oocyte by the sperm.
Let us review the stages of spermatogenesis now. At the periphery of the seminiferous tubule are the early dark spermatogonia. Their presence is not hormonally dependent. The stages of production of sperm proceed in an orderly fashion from this dark type “A” spermatogonia. First the spermatogonia become pale and then transform into a type “B” spermatogonia (Fig. 3). Spermatogenesis is a process whereby the chromatin that were originally present in the spermatogonia begin gradually to condense in preparation for the first meiotic reduction division.
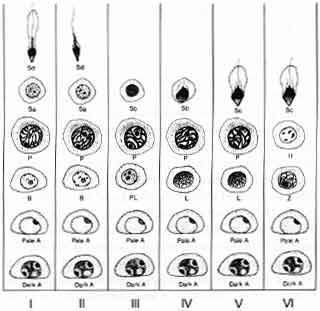
This begins with the development of the type “B” spermatogonia, the preleptotene spermatogonia, and then the leptotene spermatogonia. This early process is equivalent to prophase in the stages of oogenesis.
During the follicular phase of oogenesis, the primordial oocyte is enlarging from about 20 micrometers to 130 micrometers in size, under the influence primarily of FSH. During this process LH receptors are prepared for the preovulatory LH surge.
This LH surge is not merely a way of triggering ovulation. In fact, ovulation is a relatively minor effect of LH. The major genetic effect of LH is to initiate the resumption of meiosis, which had been arrested from infancy at prophase.
This is equivalent to what happens in the male as the spermatogonia become transformed into preleptotene, leptotene, zygotene, and pachytene spermatocytes. This is a process whereby chromosomes are condensing and preparing for the first meiotic division.
In men who have no pituitary function, the mere administration of LH or human chorionic gonadotropin (hCG) will bring spermatogenesis up to this pachytene spermatocyte stage but does not allow meiosis to complete. Meiosis cannot be completed in the spermatogenic process without FSH.
However, since infertile men with severe oligospermia or azoospermia most commonly have a normal FSH and LH level, the second reduction division (which is the usual deficiency in infertile men), does not appear to be a hormonally dependent event. This is quite similar to the female whereby the release of the second polar body requires prior FSH and LH priming but is apparently not triggered specifically by FSH or LH, but rather by the event of sperm penetration.(32)
In almost all other animals there is an orderly wave of spermatogenesis proceeding along the seminiferous tubule. At any point in the tubule you can only see one specific stage of spermatogenesis (e.g., spermatogonia, early spermatocytes, late spermatocytes, or perhaps mature sperm).
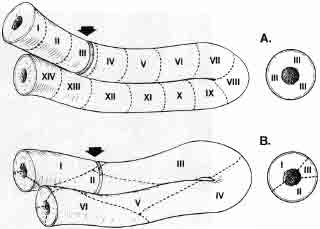
In nonprimate animals, this production of sperm occurs in an organized assembly-like fashion moving along the tubule in a histologically definable wave (Fig. 4). Only in humans and in some Great Apes is this process a rather unorderly, chaotic mosaic, so that different stages of spermatogenesis can be seen in a helter-skelter fashion occurring anywhere at any time in any area of the seminiferous tubule.
This chaotic arrangement of spermatogenesis in the human is part of the evolutionary breakdown in sperm production that accounts for human males being the most inefficient sperm producers (aside from a few Great Apes) in the entire animal kingdom.
Almost every animal that has ever been studied produces approximately 25 million sperm per gram of testicular tissue per day, whereas humans produce only about 4 million sperm per gram of testicular tissue per day.
Despite this chaotic arrangement of spermatogenesis in the human, however, it has been possible to locate specific stages of spermatogenesis where the production is deficient in fertile men, in infertile men, oligospermic men, “normal” men, and in aging men.
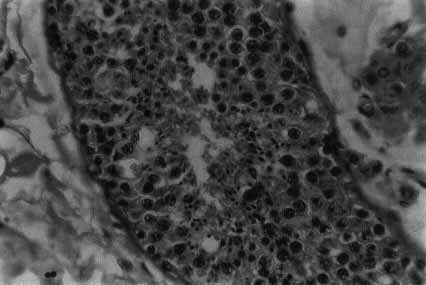
Even in so-called normal fertile men there is a moderate breakdown at meiosis. Between the easily discernible pachytene spermatocyte and the early spermatozoa (or spermatid) stage there is always some sperm loss (Fig. 5).
This is a highly significant phenomenon found only in a few animals, like humans and gorillas, where the male is reproductively very inefficient. Even in the most fertile human males there is a relatively large loss of potential spermatozoa in late meiosis that does not occur in other animals.
In oligospermic men who are infertile, this is also the most common stage where spermatogenesis is deficient. It is simply that in these infertile oligospermic men, the late meiotic breakdown is far more severe than in so-called normal fertile men.
Of course, it is true that there are a minority of infertile oligospermic males who have either Sertoli cell only syndrome with no spermatogonia, or simply vastly reduced total spermatogenesis starting from the spermatogonial level. These men generally have an elevated FSH. However, they are a distinctly small minority of the infertile male population.
Most infertile males with severe oligospermia have a normal FSH and normal or even high numbers of spermatogonia and spermatocytes, but simply little or no conversion from spermatocyte to spermatids in the second meotic division.
Spermatogenic Deficiency in Aging Men
Until recently we had a poor understanding of the effect of aging on male fertility. It was assumed that male fertility was relatively immortal because so many elderly men have been able to impregnate their wives.
However, there has been previous crude data showing a relative decrease in sperm count, and possibly fertility, in a certain percent of aging men.(26,34) According to an older study of testicle biopsy sections of men in the third and fourth decades of life, 90% of seminiferous tubules contained spermatids, but in the fifth to seventh decades of life, only 50% of the seminiferous tubules contained mature spermatids.
In men over 80 years of age only 10% of seminiferous tubules contained mature spermatids. Thus, although some seminiferous tubules continue to make sperm sufficient for impregnating the female partner as late as the ninth decade of life, the percentage of seminiferous tubules still functioning and making sperm quite distinctly decline with advancing age.
When the sperm count is reduced in young infertile men, as mentioned before, it is usually closely related to the percentage of cell loss during postprophase of meiosis (i.e., the second meiotic division).(35)
With careful, quantitative evaluations of testicular histology, sperm production rates in humans are usually closely related to the percentage of cell loss during the later stages of meiosis both in infertile as well as fertile men.(30,31,36-39)
However, in the aging testicle the situation is different. In aging men, the reduction in average daily sperm production occurs during meiosis also, but it does not occur in the late stage of meiosis.(40)
Rather, the age-related decline in daily sperm production results largely from a block to further meiosis in the early prophase stage of meiosis. To explain this in a different fashion, there is no difference between older men and younger men in the number of early primary spermatocytes per gram of testicular tissue.
However, there is a vast difference between older and younger men in the number of late spermatocytes. This is what causes the mean reduction in numbers of spermatozoa seen in a population of older men versus a similarly random population of younger men. Older men have a significant reduction ‘in potential daily sperm production between the early and the late primary spermatocyte stage, not late in meiosis as occurs more commonly in infertile men.(41,42)
Thus, the type of maturation arrest that is the most common cause of male factor infertility, and occurs to some degree in all normal men as well, is not the type of maturation arrest that appears to result from the aging process.
Rather, the aging process results in a maturation arrest at a much earlier stage of spermatogenesis, and this seems to be related to a decrease in quantity of Sertoli cells present in older men’s testicles compared to that of young men’s testicles.(43)
Since the pattern of disturbed spermatogenesis in aging men is different from that of other fertile and infertile populations, we are not able to state for certain whether this merely represents a reduction in total amount of sperm produced or a true reduction in the fertility potential of elderly men.
For example, a man who in his youth had a sperm count of 50 million per cc might perhaps in his 80s have a sperm count of 10 million per cc. If there is no specific pathological process other than aging, it is possible that his only problem is a reduction in the number of spermatozoa and not a reduction in fertility.
The answer to this question awaits the type of massive clinical study in men of varying ages equivalent to what the French reported in 1982 with women varying in ages undergoing insemination with normal donor sperm.
That is, the only definitive answer to the question of whether aging men with diminished spermatogenesis lose their fertility is to take a group of older and younger men who are mated to a homogenous population of fertile women and to determine whether the pregnancy rate or fertilization rate with IVF would be lower in those women whose husbands were older as opposed to those whose husbands were younger.
Regardless of this present lack of clarity on the issue of fertility in older men, at least it can be said with certainty that there is an age-related decline in spermatogenesis that might possibly result in a moderate decline in male fertility.
REFERENCES
- Mosher W: Reproductive impurement among currently married couples; United States 1976. Special report from Advanced Data from Vital and Health Statistics and Technology Public Health Service. #55, January 24, 1980
- Mosher WD: Factors related to infertility in the United States 1965-1976. J Sex Transm Dis July-September 117, 1985
- Mosher W: Infertility; Why Business is Booming. Am Demograph July:42-43, 1987
- Mosher WD: Fecundity and infertility in the United States 1965-1982. Advanced Data 1:1, 1985
- Federation Cecos, Schwartz D, Mayoux NJ: Female fecundity as a function of age: Results of artificial insemination in 2,193 nulliparous women with azospermic husband. N Engl J Med 306:404, 1982
- Emperaire JC, Gauzere E, Audebert A: Female fertility and donor insemination. Lancet 1:1423, 1980
- Romeo A, Musher SJ, et al: Results of in vitro fertilization attempts in women 40 years of age and older: The Norfolk Experience. Fertil Steril 47:130, 1987
- Serhal PF Craft IL: Oocyte donation in 61 patients. Lancet 1185, 1989
- Michelow MC, Bernstein J, Jacobson Mj, et al: Mother daughter in vitro fertilization triplet surrogate pregnancy J In Vitro Fert Embryo Transf 5:31, 1988
- Borrero C, Remohi J, Ord T, et al: A program of oocyte donation in gamete intrafallopian transfer. Hum Reprod 4: 275, 1989
- Rosenwak Z, Veeck LL, Liu HC: Pregnancy following transfer of in vitro fertilized donated oocytes. Fertil Steril 45:417, 1986
- Sauer NV Paulson Rj, Macaso TM, et al: Establishment of a non anonymous donor oocyte program; preliminary experience at the University of So California. Fertil Steril 52:433, 1989
- Silber Sj: The Mate. New York: Charles Scribner’s Sons Publishers, 1981, pp 11-14, 66-69
- Morley JE, Korenman SG, Mooradian AD, Kaiser FE: UCLA Geriatric grand rounds sexual clsyfunction in the elderly male. American Geriatrics Society. J Am Geriatr Soc 35:1014, 1987
- Kaiser FE, Viosca SP Morley JE, et al: Impotence and aging: Clinical and hormonal factors. J Am Geriatr Soc 36:511, 1988
- Morley JE, Kaiser FE: Sexual function with advancing age. Geriatric Medicine. Med Clin North Am 73:483, 1989
- Diokno AC, Brown MB, Herzog AR: Sexual function in the elderly Arch Intern Med 150:197, 1990
- Ware JC: Impotence and Aging. Clin Geriatr Med 5:301,1989
- Nankin HR, Calkins JH: Decrease bio-available testosterone in aging normal and impotent men. J Clin Endocrinol Metab 63:418, 1986
- Seymour Fl, Duffy C, Koerner A: A case of authenticated fertility in a man of 94. JAMA 105:423, 1935
- Becker RB: Average useful life span and causes of losses of dairy bulls. J Dairy Sci 23:548, 1940
- Becker RB, Dix Arnold PT, Sparlock AH: Production life span of dairy cattle. Bulletin of Florida Agriculture and Experimental Science #540, 1954
- Hotchkiss RS: Fertility in Men. Philadelphia: JB Lippincott, 1945
- Engle ET: Male reproductive system. In: Lansing Al (Ed): Cowdry~ Problems of Aging, 3rd ed. Baltimore: Williams & Wilkins, 1952, pp 708-729
- Bishop MWH: Aging in reproduction in the male. J Reprod Fertil 12(Suppl):65, 1970
- Johnson L, Petty CS, Neaves WB: Influence of age on sperm production and testicular weights in men. J Reprod Fertil 70: 211,1984
- Heller CG, Clermont W: Kinetics of the germinal epithelium in man. Rec Prog Horm Res 20:454, 1964
- Stemberger E, Tjioe DY: A method of quantitative analysis of human serniniferous epithelium. Fertil Steril 19:960, 1968
- Zuckerman Z, Rodriquez-Rigau LJ, Weiss DB, et al: Quantitative analysis of the serniniferous epithelium in human testicular biopsies, and the relation of spermatogenesis to sperm density Fertil Steril 30:448, 1978
- Silber SJ, Rodriquez-Rigau Lj: Quantitative analysis of testicle biopsy Fertil Steril 36:480, 1981
- Silber SJ, Patrizio P Asch RH: Quantitative evaluation of spermatogenesis by testicular histology in men with congenital absence of the vas deferens undergoing epididymal sperm aspiration. Hum Reprod 5:89, 1990
- Silber SJ: How to get Pregnant with the New Technology. New York: Warner Books, 1990
- Clermont Y: The cycle of the serniniferous epithelium in man. Am J Anat 112:35, 1963
- Sasano N, lchijo S: Vascular patterns of the human testis with special reference to its senile changes. Tohoku J Exp Med 99:269, 1969
- Johnson L, Petty CS, Neaves WB: Further quantification of human spermatogenesis: Germ cell loss during post-prophase of meiosis and its relationship to daily sperm production. Biol Reprod 29:207, 1983
- Johnson L: A re-evaluation of daily sperm output of men. Fertil Steril 37:811, 1982
- Johnson L, Petty CS, Neaves WB: A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod 22:1233, 1980
- Johnson L, Petty CS, Neaves WB: The relationship of biopsy evaluation and resticular measurements to overall daily sperm production in human testes. Fertil Steril 34:36, 1980
- Silber Sj: Reproductive Infertility Microsurgery in the Male and Female. Baltimore: Williams & Wilkins, 1984
- Johnson L: Spermatogenesis and aging in the human. J Androl 7:331, 1986
- Johnson L, Petty CS, Porter JC, Neaves WB: Germ cell degeneration during post-prophase of meiosis and serum concentrations of gonadotropins in young adult and older adult men. Biol Reprod 31:779, 1984
- Johnson L, Nguyen HB, Petty CS, Neaves WB: Quantification of human spermatogenesis: Germ cell degeneration during spermatogenesis and meiosis in testis from younger and older men. Biol Reprod 37:739, 1987
- Johnson L, Zane RS, Petty CS, Neaves WB: Quantification of the human Sertoli cell population: Its distribution, relation to germ cell numbers, and age related decline. Biol Reprod 31:785, 1984