Dr. Sherman J. Silber of The Infertility Center of Saint Louis, St Luke’s Hospital, Saint Louis, MO 63017, USA
Human Fertility – December 2010
Abstract
It is often questioned whether sperm parameters, including whether retrieved or ejaculated, have any effect on intracytoplasmic sperm injection (ICSI) results. Do severe spermatogenic defects affect embryo quality or pregnancy rate? Further, does it matter in azoospermic patients whether the sperm source is testicular or epididymal? Our studies show there is no significant difference in results with ICSI related to any sperm count parameters either with patient’s sperm or even with donor sperm. No matter how poor the sperm count, there was no difference from patients with high sperm counts nor even patients using donor sperm. There is no significant difference between results with epididymal sperm, either fresh or frozen, in comparison to results with ejaculated or donor sperm. It is obvious that in all these cycles the female partner’s age was the most important determinant of delivery rate, and the small amount of sperm is irrelevant to success rates.
Introduction
Approximately one out of every 200 men in any population (excluding those who have had a vasectomy) is azoospermic (Hull et al., 1985) (Figure 1). Approximately 20% of couples in the United States are infertile (Mosher, 1985, 1987), and approximately 25% of all infertile couples have a low sperm count (Hull et al., 1985). About 2% of infertile couples have azoospermia (Hull et al., 1985). Thus, azoospermia represents approximately 8% of the cases of male infertility, and we classify azoospermia as ‘obstructive’ and ‘non-obstructive.’
Sperm retrieval, whether for obstructive or nonobstructive azoospermia (NOA), should be a quick outpatient procedure under local anaesthesia (Silber, 2000a). The spermatic cord is injected with about 6 ml of 0.5% marcaine (bupivacaine) via a 25-gauge needle just distal to the external inguinal ring. Then an additional 2 ml of 0.5% marcaine is injected over the anterior scrotal skin in the area where a 1–2 cm incision is made and carried down to the tunica vaginalis. The testis and epididymis are then extruded and fully exposed. For testis sperm extraction (TESE) an incision is made in the tunica albuginea, and all anatomic lobules of the testis are exposed and sampled. This is a thoroughly painless clinical procedure (except for the initial injection of local anaesthetic). The patient is able to get up and walk away immediately afterward with no more pain than if he would have had a vasectomy. For obstructive cases, epididymal sperm (microsurgical epididymal sperm aspiration (MESA)) retrieval is preferred to TESE, and the data supporting this will be explained.
Needle biopsy is another alternative, but it is no less painful than the open biopsy as described above, and the open biopsy can always sample every anatomic lobule of seminiferous tubules so as to be certain that if there are any sperm, they will be found. Needle biopsy cannot accomplish this unless performed multiple times, which is then ironically more invasive and painful than the open biopsy technique, and the results much less certain.
There has been considerable unnecessary confusion about the interpretation and counting of mature spermatids in the testis biopsy. The mature spermatid always has a tail, but this is rarely seen on histological section, because the sperm head is 4 microns wide and likely to be in the cut of the microtome’s thin section, but the sperm tail is less than 1 micron in thickness and unlikely to be in the cut section of the microtome. Therefore, when viewing a histologic section, the spermatids will appear to be without tails even though with TESE, they will appear just like sperm.
Needle biopsy is another alternative, but it is no less painful than the open biopsy as described above, and the open biopsy can always sample every anatomic lobule of seminiferous tubules so as to be certain that if there are any sperm, they will be found. Needle biopsy cannot accomplish this unless performed multiple times, which is then ironically more invasive and painful than the open biopsy technique, and the results much less certain.
There has been considerable unnecessary confusion about the interpretation and counting of mature spermatids in the testis biopsy. The mature spermatid always has a tail, but this is rarely seen on histological section, because the sperm head is 4 microns wide and likely to be in the cut of the microtome’s thin section, but the sperm tail is less than 1 micron in thickness and unlikely to be in the cut section of the microtome. Therefore, when viewing a histologic section, the spermatids will appear to be without tails even though with TESE, they will appear just like sperm.
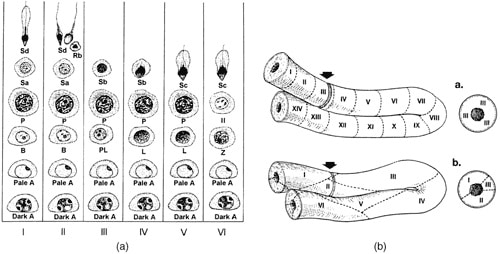
Some clinicians have attempted to use the serum follicle-stimulating hormone (FSH) level to monitor the amount of spermatogenesis. A normal FSH level in an azoospermic patient would supposedly indicate obstruction. Unfortunately, this correlation is very poor (De Kretser et al., 1974). Patients with maturation arrest causing azoospermia have a normal FSH level. Furthermore, even the tiniest number of sperm in the testis (not enough to reduce the FSH level) is adequate for successful intracytoplasmic sperm injection (ICSI). Ironically, it is the scattered mosaic arrangement of the various stages of sperma- togenesis in the human seminiferous tubule (as opposed to the orderly wave moving across the tubule in most other species) that makes quantifying the human testicular biopsy so simple. In rats, a cut through any particular seminiferous tubule shows only one particular stage (Figure 2a and b). In humans, a cut through any area of the testicle reveals a scattered array of all the various stages of spermatogenesis. Thus, in humans, unlike most other animals, it requires only 25 seminiferous tubules from anywhere in the testis for a good statistical sample of the total range of spermatogen- esis in the entire testicle.
Sperm retrieval and ICSI for obstructive azoospermia (MESA and TESE)
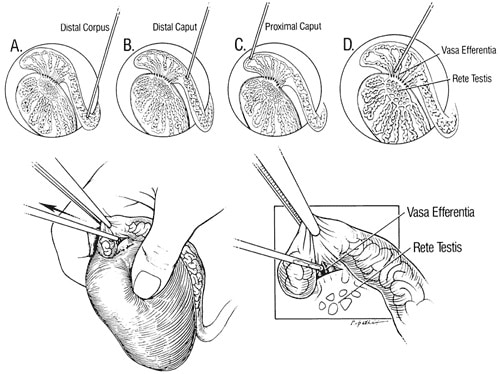
Since the first successful use of epididymal sperm aspiration and IVF for CBVAD was reported, ICSI has now made it possible for all of these men to have children (Silber et al., 1987, 1988, 1990a,b, 1994, 1997a,b; Tournaye et al., 1994). In fact, with ICSI, the pregnancy rate with microsurgical epididymal sperm retrieval (MESA) is only related to female factors (Silber et al., 1994, 1995a,b,c, 1997a,b). Frozen epididymal sperm gives results with ICSI no different from fresh, and one MESA procedure should provide enough sperm for virtually an infinite number of ICSI-IVF cycles. Under 106 to 406 magnification with an operating microscope, a 0.5-cm incision is made with microscissors into the epididymal tunic to expose the tubules in the most proximal portion of the congenitally blindending epididymis. Sperm are aspirated with a micropipette (0.7 mm/22mm; Cook Urological, Spencer, IN) on a tuberculin syringe directly from the opening in the epididymal tubule. The specimens are immediately diluted in HEPES-buffered medium, and a tiny portion is examined for motility and quality of progression. If sperm motility is absent or poor, another aspiration is made 0.5 cm more proximally. Sperm are obtained from successively more proximal regions until progressive motility is found (Figure 3).
Motile sperm are usually not obtained until the most proximal portion of the caput epididymis or vasa efferentia is reached. This is the opposite of what you would find in a normal, non-obstructed epididymis and is the cause of much confusion in the literature on this subject. In the obstructed epididymis, the most recently produced sperm are the most proximal, and therefore the most viable and motile. The distal epididymal sperm are the most senescent and clearly non-viable. Therefore, distal epididymal sperm have much greater sperm DNA fragmentation so obvious as to be readily observable under electron microscopy (Asch et al., 1992). Once the area of motile sperm is found, an aliquot of epididymal fluid is used for ICSI, and the remainder is frozen.
For obstructive azoospermia (OA) we prefer to use epididymal sperm, although testicular sperm often works just as well. One advantage of epididymal sperm as a first choice is that it freezes so easily and represents such a simple, clean, easy and indefinite supply of sperm for the laboratory to use for that particular patient, without any need for future invasive procedures. More importantly, our data now show that a higher ICSI success rate is achieved with motile epididymal sperm as opposed to less mature testis sperm.
There have been many trivial debates over how to best collect epididymal or testicular sperm from azoospermic patients for ICSI. For OA, there is usually some epididymis present no matter how severe the congenital defect. In these instances, we prefer microsurgical epididymal sperm aspiration (MESA). We do all sperm retrieval under local anaesthesia without sedation. Although the approach is microsurgical and careful, it is an outpatient procedure performed with minimal postoperative discomfort. Once the tunica vaginalis is entered, the epididymis and testicle are exposed and brought into the field of an operating microscope. The patient, indeed, can watch the whole procedure on a video monitor and should be wide-awake and comfortable. The advantage of epididymal sperm retrieval performed in this fashion is the huge number of the most motile sperm that can readily be obtained from the most proximal duct and frozen for an unlimited number of future ICSI cycles.
There is often only one specific area of the proximal epididymis where motile sperm can be retrieved, and this can be found more easily through microsurgery than via a blind needle stick (which, in truth, is a more painful than this microsurgical MESA procedure).
An important warning is that for NOA, epididymal sperm can never be retrieved, because the walls are collapsed and there is no obstruction to allow epididymal sperm collection to take place. For NOA, an open testicular biopsy, performed under the microscope, must be performed under the same type of local anaesthetic, with the patient wide-awake, and with minimum postoperative discomfort.
Testicular sperm extraction (TESE) for non-obstructive azoospermia

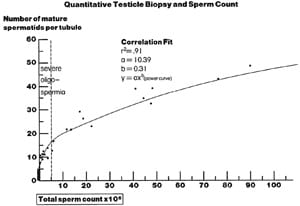
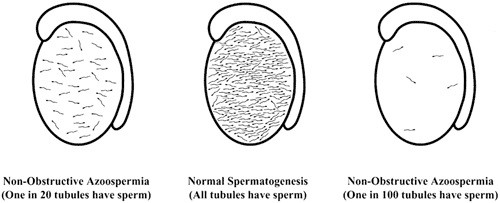
Shortly after introducing sperm retrieval for OA, we made the observation that even in men with the most severe spermatogenic defects, causing complete azoospermia, there were often a very minute number of sperm sparsely present in an extensive testicular biopsy, and these occasional testicular sperm could be used for ICSI (Devroey et al., 1995a,b; Silber et al., 1995a,b,c, 1996, 1997a,b; Silber, 1998). We coined this procedure testicular sperm extraction (TESE). This approach was based on quantitative studies of spermatogenesis dating back to the late 1970s (Steinberger & Tjioe, 1968; Zukerman et al., 1978; Silber & Rodriguez-Rigau, 1981; Silber et al., 1990a,b). The examination of the testicular histology of azoospermic, oligospermic and normospermic men shows that the number of sperm in the ejaculate is directly correlated to the number of mature spermatids found quantitatively in the testis. The average mature spermatid count per tubule in a large number of tubules is predictive of the sperm count in the ejaculate. Intriguingly, about 60% of patients with complete azoospermia have a few mature spermatids in their testis histology (Figure 4).
An extremely diminished quantity of sperm production in the testis will result in absolute absence of sperm in the ejaculate even though there is some sperm being produced in the testicle. There is a minimal quantitative threshold of sperm production that is necessary before any sperm can actually ‘spill over’ into the ejaculate. Thus, severe oligospermia, which is readily treated with ICSI, is just a quantitative variant of azoospermia where more than three mature spermatids per tubule are found in the testis. There is some minute presence of spermatogenesis in 60% of azoospermic men. However, the amount of spermatogenesis is below the threshold (three mature spermatids per tubule) necessary for these few sperm to ‘spill over’ into the ejaculate (Silber et al., 1997a,b).
Microsurgical TESE
When extensive multiple biopsies from every area of the testis are performed in an effort to find sufficient sperm for TESE, a great deal of testicular damage can result, and may limit ‘successful’ patients to only one attempt (Tournaye et al., 1996, 1997). An attempt to limit damage by using multiple needle sticks rather than open biopsy to obtain sperm for ICSI is just as invasive and quite risky as well (Craft et al., 1997). Furthermore, control studies have shown that for difficult cases of NOA, where spermatogenesis is very meager, needle biopsy is much less likely to find the rare foci of spermatogenesis than open biopsy (Friedler et al., 1997; Rosenlund et al., 1998).
We studied the distribution of spermatogenesis in azoospermic men, and have outlined a microsurgical approach to TESE that minimises tissue loss and pain, and makes TESE very easily repeatable for an indefinite number of cycles. Knowledge of the distribution of spermatogenesis and use of microsurgical technique helps to prevent testicular damage and post-operative pain, making multiple repeat TESE procedures (if needed) safe and reliable (Figure 5) (Silber et al., 1997a,b; Silber 2000b).
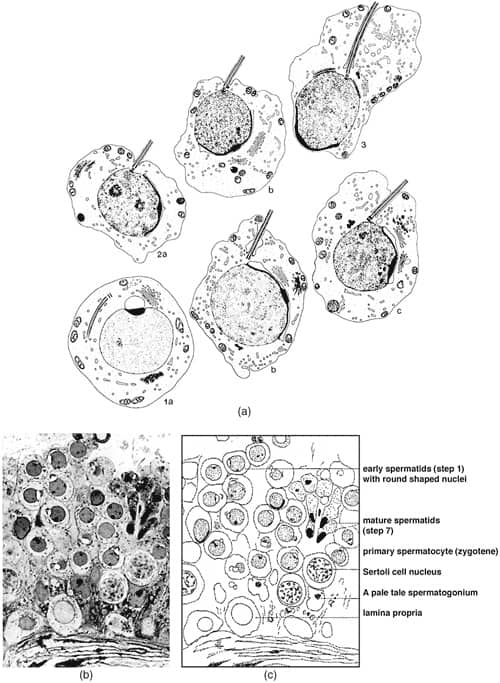
The solution to cases where there are no sperm to be seen on TESE is not to look for ’round spermatids’ (Silber & Johnson, 1998; Silber et al., 2000). We never see round spermatids in the absence of mature spermatids, which at TESE are what just appear to be sperm (Figure 6a–c) (Silber & Johnson, 1998; Silber et al., 2000; Holstein & Roosen-Runge, 1981). The solution is to search for the few sperm with tails that are very sparsely and diffusely present.
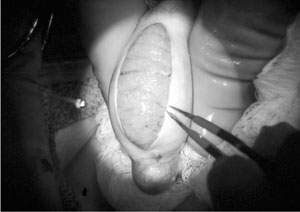
Technique of microsurgical tese procedure
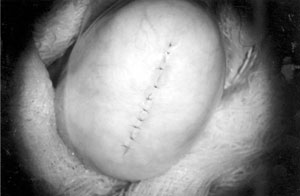
There has been a great deal of confusion created by the term ‘microsurgical TESE’. One extremely damaging approach is the so-called ‘micro-dissection’. In this approach, the entire testis is exposed, not just the periphery, and every millimetre is dissected looking for ‘dilated’ tubules. The idea is to try to limit somniferous tubule loss, but in truth, the opposite results, and many of these cases result in virtual castration (Schlegel & Chang, 1991; Schlegel & Su, 1997; Schlegel et al., 1997; Schlegal 1999). The safe and equally effective alternative microsurgical approach is as follows (Silber 2000a,b). Under the operating microscope, a longitudinal incision is made in the tunica albuginea exposing all anatomic lobules of the testis. Tissues from only the peripheral loops of the seminiferous tubules are sampled. This avoids damage to the testis and yet samples every corner from which sperm might be present. The tunica albuginea is closed with 9-0 nylon interrupted sutures, after meticulous haemostasis with microbipolar forceps (Figures 7 and 8). This prevents any increase in intratesticular pressure, resulting in minimal pain and no subsequent atrophy.
Of the total cases subjected to microsurgical TESE for NOA, about 60% yield sperm sufficient for ICSI. In maturation arrest, often a larger amount of testicular tissue has to be removed because all tubules are normal size, whereas with Sertoli cell only, the tubules are very thin. Nonetheless, in either case, minimal damage is incurred with micro TESE because blood supply is not interrupted, microscopic bleeders are meticulously coagulated, and tunica albuginea is not encroached upon because of the closure with 9–0 nylon interrupted stitches. Consequently there is no increase in intra-testicular pressure (Figures 7 and 8). This results in no testicular damage, and minimal pain.
Of the total cases subjected to microsurgical TESE for NOA, about 60% yield sperm sufficient for ICSI. In maturation arrest, often a larger amount of testicular tissue has to be removed because all tubules are normal size, whereas with Sertoli cell only, the tubules are very thin. Nonetheless, in either case, minimal damage is incurred with micro TESE because blood supply is not interrupted, microscopic bleeders are meticulously coagulated, and tunica albuginea is not encroached upon because of the closure with 9–0 nylon interrupted stitches. Consequently there is no increase in intra-testicular pressure (Figures 7 and 8). This results in no testicular damage, and minimal pain.
Our direct mapping gives evidence for a diffuse rather than regional distribution of spermatogenesis in NOA (Silber & Rodriguez-Rigau, 1981; Silber et al., 1997a,b; Silber 2000a,b). Furthermore, the variation in sparseness of spermatogenesis verified by observation of contiguous strips of testicular tissue, explains why a single random biopsy may or may not yield sperm, and why with OA removal of very small amounts of tissue blindly with a needle might have an success rate, but has a very low success rate with NOA.
The formidable testicular deterioration that has been observed with overly aggressive TESE procedures is caused by either direct interference with microvascular supply of the seminiferous tubules or even more commonly, increased intratesticular pressure caused by minor amounts of bleeding within the enclosed tunica albuginea. The tunica albuginea is a very non-flexible enclosure. A small degree of intratesticular bleeding causes a noticeable increase in intratesticular pressure. This can be readily observed by anybody doing conventional, multiple testicle biopsy samplings for TESE. Furthermore, the closure of open biopsies with the usual non-microsurgical suture, particularly in a running fashion with conventional TESE, further compromises the intratesticular volume and thereby adds to the increased pressure.
ICSI–IVF results with testis sperm vs. epididymal sperm vs. ejaculated sperm
In our IVF programme, all oocytes undergo ICSI without any selection bias based on semen parameters or other sperm testing. This afforded an excellent opportunity to evaluate the degree to which ICSI–IVF results are or are not affected by he degree of spermatogenic defect. One thousand eight hundred forty-nine consecutive unselected ICSI cycles with ejaculated sperm were analysed retrospectively for success rates based on sperm count ranging from below 2 million/ml to greater than 20 million/ml to donor sperm. These results were compared to ICSI success rates for 719 azoospermic cycles using testicular sperm versus using epididymal sperm. For NOA, only testicular sperm could be obtained (TESE). For OA, epididymal sperm (MESA) was used in most cases, but in many cases testicular sperm (TESE) was used for obstructive (OA). MESA and TESE cases were divided and analysed according to fresh and frozen, as well as OA versus NOA categories. Results were analysed with chi square, and were reported and reviewed at the British Andrology Society Meeting, Belfast, Northern Ireland, November 2009.
There is no significant difference in results with ICSI related to any sperm count parameters either with patient’s sperm or even with donor sperm. No matter how poor the sperm, there was no difference.
Of course, the question arises as to why there have been instances of poorer results using epididymal sperm (MESA) in other centres (Tournaye et al., 1994). The reason for this is the paradox that most centres retrieve distal epididymal sperm under the mistaken notion (derived from the physiology of the non-obstructed state) that the distal sperm will be more mature and, therefore, of better quality. In fact, most of the epididymal sperm in the obstructed state will be non-motile because of degenerative senescence. Blind needle stick PESA usually does not locate the most proximal and most motile non-degenerative sperm. That is the reason for a microsurgical approach (MESA) to epididymal sperm retrieval.
References
- Asch, R.H., Patrizio, P., & Silber, S.J. (1992). Ultrastructure of human sperm in men with congenital absence of the vas deferens: clinical implications. Fertility & Sterility, 58, 190–193.
- Craft, I., Tsirigotis, M., Courtauld, E., & Farrer-Brown, G. (1997). Testicular needle aspiration as an alternative to biopsy for the assessment of spermatogenesis. Human Reproduction, 12, 1483–1487.
- De Kretser, D.M., Burger, H.G., & Hudson, B. (1974). The relationship between germinal cells and serum FSH levels in males with infertility. Journal of Clinical Endocrinology and Metabolism, 38, 787–793.
- Devroey, P., Liu, J., Nagy, Z., Goosens, A., Tournaye, H., Camus, M., et al. (1995a). Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non- obstructive azoospermia. Human Reproduction, 10, 1457–1460.
- Devroey, P., Silber, S., Nagy, Z., Liu, J., Tournaye, H., Joris, H., et al. (1995b). Ongoing pregnancies and birth after intracytoplasmic sperm injection with frozen-thawed epididy- mal spermatozoa. Human Reproduction, 10, 903–906.
- Friedler, S., Raziel, A., Strassburger, D., Soffer, Y., Komarovsky, D., & Ron-el, R. (1997). Testicular sperm retrieval by percutaneous five needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia. Human Reproduction, 12, 1488–1491.
- Holstein, A.F. & Roosen-Runge, E.D., Eds. (1981). Atlas of human spermatogenesis. Berlin: Grosse Verlag.
- Hull, M.G.R., Glazener, C.M.A., Kelly, M.J., Conway, D.I., Foster, P.A., Hinton, R.A., et al. (1985). Population study of causes treatment and outcome of infertility. British Medical Journal, 291, 1693–1697.
- Mosher, W.D. (1985). Fecundity and infertility in the United States 1965–1982. Advance Data, 1, 1.
- Mosher, W. (1987). Infertility: why business is booming. American Demographics, 1987, 42–43.
- Rosenlund, B., Kvist, U., Ploen, L., Rozell, B.L., Sjoblom, P., & Hillensjo, T. (1998). A comparison between open and percutaneous needle biopsies in men with azoospermia. Human Reproduction, 13, 1266–1271.
- Schlegel, P. & Chang, T. (1991). The testis, epididymis and ductus deferens. In: Walsh, P., Retik, A., Stamey, T., & Vaughan, E., (Eds.), Campbell’s urology, 6th ed. Philadelphia: Saunders, p 190.
- Schlegel, P.N. & Su, L. (1997). Physiologic consequences of testicular sperm extraction. Human Reproduction, 12, 1688– 1692.
- Schlegel, P., Palermo, G., Goldstein, M., Menedez, S., Zaninovic, N., Veeck, L., et al. (1997). Testicular sperm extraction with intracytoplasmic sperm injection for non-obstructive azoos- permia. Urology, 49, 435–440.
- Schlegel, P. (1999). Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision.Human Reproduction, 14, 131–135.
- Silber, S.J. (1984). Reproductive infertility microsurgery in the male and female. Baltimore, MD: Williams & Wilkins, Waverly Press, Inc.
- Silber, S., Ord, T., Borrero, C., Balmaceda, J., & Asch, R. (1987). New treatment for infertility due to congenital absence of vas deferens. The Lancet, 330, 850–851.
- Silber, S.J. (1998). Intracytoplasmic sperm injection (ICSI) today: a personal review. Human Reproduction, 13, 208–218.
- Silber, S.J. (2000a). Evaluation and treatment of male infertility. Clinical obstetrics and gynecology, Vol. 43. Blanco, J.D. & Keye, R.K., (guest eds.), p 854–888. Lippincott, Williams & Wilkins.
- Silber, S.J. (2000b). Microsurgical testicular sperm extraction and the distribution of spermatogenesis in non-obstructive azoospermia. Human Reproduction, 15, 2278–2284.
- Silber, S.J. & Johnson, L. (1998). DEBATE: Are spermatid injections of any clinical value? ROSNI and ROSI revisited.Human Reproduction, 13, 509–523.
- Silber, S.J. & Rodriguez-Rigau, L.J. (1981). Quantitative analysis of testicle biopsy: determination of partial obstruction and prediction of sperm count after surgery for obstruction. Fertility & Sterility, 36, 480–485.
- Silber, S.J., Balmaceda, J., Borero, C., Ord, T., & Asch, R. (1988). Pregnancy with sperm aspiration from the proximal head of the epididymis: a new treatment for congenital absence of the vas deferens. Fertility & Sterility, 50, 525–528.
- Silber, S.J., Devroey, P., Tournaye, H., & Van Steirteghem, A.C. (1995a). Fertilizing capacity of epididymal and testicular sperm using intracytoplasmic sperm injection (ICSI). Reproduction, Fertility, and Development, 7, 281–293.
- Silber, S.J., Johnson, L., Verheyen, G., & Van Steirteghem, A. (2000). Round spermatid injection. Fertility & Sterility, 73, 897–900.
- Silber, S.J., Nagy, Z., Devroey, P., Camus, M., & Van Steirteghem, A.C. (1997a). The effect of female age and ovarian reserve on pregnancy rate in male infertility: treatment of azoospermia with sperm retrieval and intracytoplasmic sperm injection. Human Reproduction, 12, 2693–2700.
- Silber, S.J., Nagy, Z., Devroey, P., Tournaye, H., & Van Steirteghem, A.C. (1997b). Distribution of spermatogenesis in the testicles of azoospermic men: the presence of absence of spermatids in the testes of men with germinal failure.Human Reproduction, 12, 2422–2428.
- Silber, S.J., Nagy, Z.P., Liu, J., Godoy, H., Devroey, P., & Van Steirteghem, A.C. (1994). Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Human Reproduction, 9, 1705– 1709.
- Silber, S.J., Nagy, Z., Liu, J., Tournaye, H., Lissens, W., Ferec, C., et al. (1995b). The use of epididymal and testicular spermatozoa for intracytoplasmic sperm injection: the genetic implica- tions for male infertility. Human Reproduction, 10, 2031–2043.
- Silber, S.J., Ord, T., Balaceda, J., Patrizio, P., & Asch, R.H. (1990a). Congenital absence of the vas deferens: The fertilizing capacity of human epididymal sperm. New England Journal of Medicine, 323, 1788–1792.
- Silber, S.J., Patrizio, P., & Asch, R.H. (1990b). Quantitative
evaluation of spermatogenesis by testicular histology in men with congenital absence of the vas deferens undergoing epididymal sperm aspiration. Human Reproduction, 5, 89–93. - Silber, S.J., Van Steirteghem, A.C., Liu, J., Nagy, Z., Tournaye, H., & Devroey, P. (1995c). High fertilization and pregnancy rate after intracytoplasmic sperm injection with spermatozoa obtained from testicle biopsy. Human Reproduction, 10, 148– 152.
- Silber, S.J., Van Steirteghem, A.C., Nagy, Z., Liu, J., Tournaye, H., & Devroey, P. (1996). Normal pregnancies resulting from testicular sperm extraction and intracytoplasmic sperm injection for azoospermia due to maturation arrest. Fertility & Sterility, 66, 110–117.
- Steinberger, E., & Tjioe, D.Y. (1968). A method for quantitative analysis of human seminiferous epithelium. Fertility & Sterility, 19, 960–970.
- Tournaye, H., Devroey, P., Liu, J., Nagy, Z., Lissens, W., & Van Steirteghem, A. (1994). Microsurgical epididymal sperm aspiration and intracytoplasmic sperm injection: a new effective approach to infertility as a result of congenital bilateral absence of the vas deferens. Fertility & Sterility, 61, 1045–1051.
- Tournaye, H., Liu, J., Nagy, P.Z., Camus, M., Goossens, A., Silber, S., et al. (1996). Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Human Reproduction, 11, 127–132.
- Tournaye, H., Verheyen, G., Nagy, P., Ubaldi, F., Goossens, A., Silber, S., et al. (1997). Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Human Reproduction, 12, 80–86.
- Zukerman, Z., Rodriguez-Rigau, L., Weiss, D.B., Chowdury, L.J., Smith, K.D., & Steinberger, E. (1978). Quantitative analysis of the seminiferous epithelium in human testicle biopsies and the relation to spermatogenesis to sperm density.Fertility & Sterility, 30, 448–455.
