S. Boutellea, K. Lenahanb, R. Krisherc, K. L. Baumana, C.S.Asaa*, S. Silberb
a Research Department, Saint Louis Zoo, St. Louis, MO 63110 United States; b St. Luke’s Hospital, Chesterfield, MO 63017; c University of Illinois, Urbana, IL 61801 USA, *Corresponding author. Tel.: 314-646-4523; fax: 314-646-5534.
Theriogenology, October 2010
Download PDF version of this article
Careful genetic management, including cryopreservation of genetic material, is central to conservation of the endangered Mexican gray wolf. We tested a technique, previously used to vitrify human and domestic animal oocytes, on oocytes from domestic dogs as a model and from the endangered Mexican wolf. This method provided a way to conserve oocytes from genetically valuable older female Mexican wolves as an alternative to embryos for preserving female genes. Oocytes were aspirated from ovaries of 36 female dogs in December and March (0 to 65 oocytes per female) and from six female wolves (4 to 73 per female) during their physiologic breeding season, or following stimulation with the GnRH agonist deslorelin. Oocytes from dogs were pooled; half were immediately tested for viability and the remainder vitrified, then warmed and tested for viability. All oocytes were vitrified by being moved through media of increasing cryoprotectant concentration, placed on Cryotops, and plunged into liquid nitrogen. There was no difference in viability (propidium iodide staining) between fresh and vitrified, warmed dog oocytes (65.7 and 61.0%, respectively, P = 0.27). Oocyte viability after warming was similarly assessed in a subset of wolves (4 to 15 oocytes from each of three females; total 29 oocytes). Of these, 57.1% of the post-thaw intact oocytes were viable, which was 41.4% of all oocytes warmed. These were the first oocytes from a canid or an endangered species demonstrated to have maintained viability after vitrification and warming. Furthermore, our results demonstrated that vitrification of oocytes with the Cryotop technique was an option for preserving female gametes from Mexican wolves for future use in captive breeding programs, although in vitro embryo production techniques must first be developed in canids for this technique to be used.
Introduction
The Mexican gray wolf (Canis lupus baileyi), listed in 1976 by the U.S. Fish and Wildlife Service (USFWS) as an endangered subspecies of the gray wolf, provides an excellent example of the successful use of captive breeding in species recovery. Considered extinct in the wild at the time of listing, reintroduction relied solely on wolves produced through captive breeding. The current captive population of Mexican wolves numbers 340 individuals maintained in 47 zoos and related facilities in the USA and Mexico [1]. However, because these wolves all trace to only six or seven original founders [2], that is, genetic ancestors of the entire Mexican wolf population, careful genetic management is required. Selection of breeding pairs seeks to maintain or increase gene diversity by considering mean kinship (how many relatives exist in the current population) and by avoiding inbreeding [1].
To preserve genes from this limited population, a frozen semen bank was created in 1991 at the Saint Louis Zoo, under the auspices of the USFWS Mexican wolf recovery program. At present, this semen bank holds samples from 65 individual Mexican wolves. However, until very recently, the only method for preserving female gametes was by embryo cryopreservation, which does not permit as much genetic flexibility as possible with egg and sperm cells, because the genetic match must be made at the time the embryo is created for cryopreservation, with currently available sperm sample(s). Having the option to fertilize ova from these females with sperm from more males than those currently living is important to optimize genetic management. The ability to cryopreserve ova rather than embryos from genetically valuable Mexican wolf females for future use in in vitro fertilization would provide an extremely valuable tool to population managers.
Vitrification has been successful with ova from a variety of domestic and laboratory species as well as humans (cow [3], horse and cow [4], pig [5], cat [6], human [7–11]), but has not yet been applied to endangered species recovery programs. Although successful in vitro maturation and fertilization protocols have not been established for canids, vitrification of oocytes could rescue genes from female Mexican wolves, especially from aging females that have produced few or no offspring, for future use. Given the tremendous advances in in vitro embryo production systems in recent years and, in particular, the interest in in vitro embryo techniques for application to domestic dogs, these technologies are likely to become available, so that frozen eggs can be used when the genes represented by these samples are needed for genetic population management in the future.
The objective of this study was to establish a protocol to cryopreserve oocytes from Mexican wolves. Conducting properly controlled studies with wolf oocytes was not possible, because we were required by USFWS and the AZA Mexican Wolf SSP to minimize wastage of the limited number of oocytes from these genetically valuable wolves and the protocols necessary to evaluate oocyte viability either before or after vitrification rendered them non-viable. To address this problem, we used oocytes from domestic dogs to conduct a controlled comparison of the effects of vitrification on subsequent oocyte viability. Domestic dogs are an appropriate model species for wolves, since wolves are considered to be their ancestors [12].
Materials and methods
Animals: dogs
Ovaries were obtained from various breeds of dogs on two independent occasions in December (N = 16) and March (N = 20) following elective ovariohysterectomy at two local veterinary clinics. Based on owner information, all females were adults except one, assumed prepubertal because it was less than 1 y of age and estrus had not been observed. For the adult dogs, day of cycle at time of surgery was not known, but none had been treated with products for contraception or stimulation of estrus or ovulation. Because dogs do not reproduce seasonally, these females could have been in any point of the reproductive cycle at the time of surgery.
Animals: wolves
The Association of Zoos and Aquariums (AZA) Mexican Wolf Species Survival Program (SSP) and the USFWS Mexican Wolf Recovery Program identified individual female wolves for oocyte preservation that, because of advancing age or illness, were unlikely to reproduce naturally. Females were selected because of their genetic value to the program, i.e., the importance of preserving their genes, rather than to optimize oocyte quality or quantity. The procedures were approved by the Saint Louis Zoo Institutional Animal Care and Use Committee.
Ovaries were obtained from five Mexican gray wolves and one generic gray wolf (F419) between late January and mid-March, their natural breeding season [1]. Two females (Table 1) were treated with Ovuplant® (Peptech Animal Health, New South Wales, Australia), which contains 2.1 mg of the GnRH agonist deslorelin, to stimulate follicle growth before surgery, which was scheduled to occur before predicted ovulation. Although marketed for ovulation induction in domestic mares, Ovuplant® has been used successfully to induce estrus and ovulation in domestic dogs [13] and in wolves [14]. One female (Table 1) had been treated 5 mo previously with Suprelorin® (Peptech Animal Health), which also contained deslorelin (4.7 mg), but in a slow-release implant matrix, designed for 6-mo release and used for contraception. The mode of action of Suprelorin® is to first stimulate pituitary gonadotrophs, so estrus and ovulation often occur before down-regulation is achieved. She was included in the study because she developed pyometra, necessitating ovariohysterectomy, and a decision was made to try to salvage oocytes. The remaining females were not treated with either Ovuplant® or Suprelorin®.
The wolves were housed at various zoos and captive breeding facilities in the USA (Table 1), which in four cases necessitated shipment of ovaries by air to our laboratory in St. Louis immediately after ovariectomy. For the other two female wolves, aspiration immediately followed surgery; in one case the team traveled to Minnesota and in the other, the female was brought to the Saint Louis Zoo for ovariectomy.
Shipment of dog ovaries
Ovaries, still in the bursa, were transported by car to the Saint Louis Zoo for oocyte aspiration and vitrification on the same day surgery was performed. From the time of surgical removal, ovaries were kept moist with warm sterile saline and placed in either 50 mL conical tubes or directly into a pre-warmed thermos for transfer to St. Louis. The interval from surgery to aspiration ranged from 2 to 5 h and the temperature in the container did not decrease below 28°C by the time of arrival.
Shipment of wolf ovaries
Immediately after surgical removal, ovaries were left in the bursa and wrapped in gauze squares soaked in warm sterile saline, secured in separate zip-lock plastic bags, and placed in a 250 mL plastic cup. The cup was placed in an insulated container (Equitainer: Hamilton Research Inc., South Hamilton, MA, USA) along with a gel-pack heated to approximately 38°C. The Equitainer was shipped same-day air cargo to Saint Louis, Missouri, with a resulting transit time from surgery to aspiration of 5.5 to 8.5 h. On arrival, the thermometer in the bag containing the ovaries verified that the temperature had not dropped more than 10°C.
Oocyte recovery: dog and wolf
On arrival in the laboratory, each ovary was removed from the surrounding bursa with a scalpel, and any structures (e.g., follicles, ovulation sites, corpora lutea) were noted. Aspiration was performed using a 20-g needle with a 3-mL syringe for ovaries from all but two wolves, when a vacuum pump (Cook Vmar5000: Cook Veterinary Products, Bloomington, IN, USA) was used at 2.8 mm Hg. The aspirated follicular fluid was collected into a 15 mL conical tube containing a wash solution that consisted of HEPES medium (Modified HTF Medium with Gentamicin-HEPES (10 µg/mL gentamicin, 21 mM HEPES buffer, 4 mM sodium bicarbonate): Irvine Scientific, Santa Ana, CA, USA) plus Synthetic Serum Substitute (SSS: Irvine Scientific). This wash medium (HEPES) was used throughout the study, including vitrification and thawing steps.
Oocyte selection
Follicular fluid with wash solution was transferred into 35-mm Petri dishes (Falcon 1008, Becton Dickinson, Franklin Lakes, NJ, USA) to search for oocytes. For all females, oocytes were examined for an intact zona pellucida and homogeneous cytoplasm. Due to the darkness of the cytoplasm, no further morphological assessment was possible (e.g., presence of vacuoles or pits). Oocytes were then counted and placed in a new wash solution on a warm plate. Once all oocytes were located and selected, the number to be vitrified was determined. Selection was based upon traditional morphological criteria, including an intact zona pellucida, homogeneous cytoplasm and compact cumulus cell mass, which are considered to be indicators of oocyte meiotic competence [15].
Because oocyte banking for the one non-endangered, gray wolf (419) was less critical, her oocytes were the first of those from wolves to be vitrified. In that case only, conditions were optimized for successful vitrification, rather than for eventual culture, by removing cumulus cells, because cumulus cell mass has been thought to interfere with the vitrification process. Oocytes were exposed to 60 U hyaluronidase for 30 s, and then cleaned mechanically with pulled glass pipettes. However, cumulus cells are critical for oocyte in vitro maturation [16], and more recent studies indicate that vitrification of cumulus oocyte complexes can work effectively for oocyte maturation [17]. Therefore, for oocytes from all dogs and the rest of the wolves, the corona cell layer associated with each oocyte was left intact to improve chances for future maturation and fertilization.
All selected oocytes from wolves were vitrified to preserve as many gametes as possible. However, with the dog gametes, all selected oocytes on each collection day were pooled then the pool was separated into two fractions: half (70) were immediately mounted and stained to assess viability without vitrification, whereas the remaining oocytes (75) were vitrified and later warmed to determine the effects of the vitrification process on oocyte viability.
Vitrification
For the vitrification procedure, oocytes were held at room temperature and protected from light. To prepare both dog and wolf oocytes for vitrification, each oocyte was first placed in a wash solution droplet, held at ambient temperature and then exposed to solutions with increasing concentrations of ethylene glycol (Sigma Aldrich, St. Louis, MO, USA) plus DMSO (dimethyl sulfoxide: Sigma Aldrich) in HEPES medium. Oocytes remained in each droplet for 3 min until reaching a final concentration of 7.5% ethylene glycol and 7.5% DMSO in HEPES with 20% SSS. Oocytes were then moved to another 35-mm petri dish to a final exposure of 15% ethylene glycol, 15% DMSO and 0.5 M sucrose solution in HEPES with 20% SSS using the method developed by Kuwayama and colleagues [7–9,17]. All vitrification media were prepared prior to oocyte collection and filtered using 0.22 Millex PF filter (Millipore, Billerica, MA, USA). Three to five oocytes were exposed for less than 1 min in the final vitrification solution and then transferred to Cryotops (Kitazako Supply Co., Fujinomiya, Japan) and immediately plunged into liquid nitrogen. Cryotops were then capped, placed on canes and transferred to liquid nitrogen tanks for storage.
Oocyte warming and viability testing
The effect of vitrification on dog oocytes was evaluated by comparing viability of matched subsets of oocytes: fresh vs. vitrified and warmed. In addition, a small number of vitrified oocytes from a few wolves were warmed for post-vitrification viability assessment. For the wolves, the females and the number of oocytes to be sacrificed per female was determined by the AZA Mexican Wolf SSP, based primarily on relative genetic value of the female, since such testing permanently removed the oocyte from the gene bank.
For vitrified oocytes, following removal of the protective cap while still submerged in liquid nitrogen, each Cryotop with oocytes to be warmed was transferred directly into a small culture dish containing 5 mL thawing solution (1M sucrose in HEPES medium with 20% SSS) warmed to 37°C. After 1 min in the thawing solution oocytes were transferred into droplets with decreasing concentrations of 0.5 M sucrose to 0.2 M sucrose in 2 min increments, and finally into wash solution at room temperature [8,18].
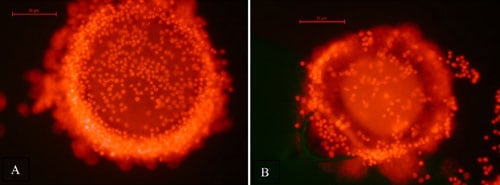
Viability of all oocytes was assessed using a standard procedure [19,20]. Oocytes were placed into DPBS supplemented with 0.1% polyvinyl alcohol and 100 µg/mL propidium iodide (PI; Sigma-Aldrich) and incubated in the dark at room temperature for 15 min. Each oocyte was examined in the staining solution using a fluorescent microscope. Oocytes that stained red (PI positive) due to disruption of the plasma membrane and passage of the PI into the cell indicated the oocyte was dead. Viable cells did not stain with any red fluorescence (PI negative), indicating plasma membrane integrity (Figure 1). After viability staining, oocytes were mounted on a glass slide under a cover slip, fixed in 3:1 acetic acid:ethanol, stained with aceto-orcein and chromatin visualized using phase contrast microscopy to evaluate nuclear maturation status.
Statistical analysis
Oocyte viability was analyzed using a balanced ANOVA. Data were coded as 0 or 1 (either intact/lysed or viable/not viable, depending on the analysis). For analysis of differences between intact and viable oocytes among females, “female” was included in the model as a fixed factor. Differences were determined by Bonferroni multiple-comparison test. For comparison of viability prior to vitrification and following warming of dog oocytes, time of assessment (i.e., pre- or post-vitrification) was included in the model as a fixed factor and replicate as a random factor. Significance was calculated as P < 0.05.
Results
Results: dog
There was no difference (P = 0.27) in viability of dog oocytes prior to and following vitrification using the Cryotop method(Table 2). During the warming process, some were lost or lysed, leaving 59 intact oocytes for viability comparison to the fresh oocytes. The decline in viability following vitrification was only 5%, (66% live prior to vitrification and 61% live following vitrification and warming) a non-significant difference. No differences in percent viability between source of ovaries or month of collection were observed, nor did viability results from the prepubertal dog differ from those from the adults. The number of oocytes recovered from the adult dogs ranged from 0 to 16 per ovary (ovaries were not maintained separately by dog, necessitating counts per ovary rather than per dog). However, the ovaries of the one prepubertal dog, which was sent separately, yielded a total of 65 oocytes.
Results: wolf
In general, more oocytes were retrieved from the ovaries of female wolves stimulated with the short-acting GnRH agonist Ovuplant, with one notable exception, female 516, whose ovaries yielded the largest number, despite not being stimulated(Table 3). The number of oocytes aspirated per female did not appear related to the date of collection (i.e., point during the breeding season) nor to female age. The smallest number of oocytes was recovered from female 741, treated with the long-acting GnRH agonist Suprelorin for contraception the previous October. She had ovulated, perhaps following that stimulation, since corpora lutea (CL) were identified. However, her reproductive tract had been removed because of pyometra, which also may have affected ovarian dynamics. Although there were five CL or corpora hemorrhagica (CH) on the ovaries of female 435, they still yielded 47 oocytes, but she had been stimulated with Ovuplant. None of the other females appeared to have ovulated before ovariectomy.
A total of 29 oocytes from three different females (one grey wolf and two Mexican wolves) were used to assess viability after vitrification and warming. Of these, eight lysed during the thawing process, leaving 21 intact oocytes for viability analysis (Table 4). There was no difference among females in the percentage of intact oocytes after warming (P > 0.05). Of the intact oocytes, nine (42.9%) were judged PI positive (dead), whereas 12 (57.1%) were PI negative (alive) (Table 4; Figure 1).
There was no difference in the percentage of live oocytes among female wolves, either as a percentage of intact oocytes after warming or of total vitrified oocytes (P > 0.05). Orcein stain revealed that all oocytes examined remained in the immature, germinal vesicle (GV) stage of meiosis, demonstrating that spontaneous nuclear maturation did not occur in either dog or wolf during aspiration, vitrification or thawing.
Discussion
Oocyte vitrification by the Cryotop method was successful, based on viability rates following thawing, for both dogs and wolves. To our knowledge, this is the first report of oocytes vitrified from any canid or from an endangered species. The percentage of viable, intact oocytes after thawing was similar for dogs and wolves (61.0 and 57.1%, respectively), suggesting that dog oocytes can serve as an adequate model for wolves. In fact, the percentage for wolves might be expected to be lower, because all the wolves were aged, whereas the dogs were of varying ages and one was prepubertal. As previously reported, fewer oocytes of lower quality were recovered from older females in dogs [21]. Nevertheless, the close agreement of the percentages of viable oocytes from the dogs and wolves suggested that this method of vitrification could be considered successful in maintaining oocyte viability in both species.
There was considerable variability in the number of oocytes recovered from each wolf. Again, their advanced ages provided one explanation [21,22]. All female wolves in this study were in the older age range (10 to 13 y) except one (F741: 7 y). She had been treated with a long-acting GnRH agonist contraceptive 5 mo before ovariectomy that had apparently stimulated ovulation, judging by the six CL at the time of aspiration, and that was likely preventing follicle development via pituitary gonadotroph down-regulation. In addition, two other females (F419: 10 y; F435: 13 y) had been stimulated with a short-acting GnRH agonist implant to induce follicle growth. They did have more oocytes than two of the females in natural estrus (F188: 12 y; F204: 11 y), yet 11-y-old F516 in natural estrus had 73 oocytes, by far the most recovered from any of the wolves. The number of oocytes recovered from each adult dog ovary varied (0–16), although not as widely as for the wolves. In all but one case (the prepubertal dog), their exact ages were not known, but it is interesting to note, though, that the prepubertal dog ovaries yielded 65 oocytes.
Another factor that could have influenced oocyte recovery was the reproductive stage of female. All wolf procedures were performed during their breeding season, mid-January through early March [1]. However, two females had already ovulated, as evidenced by the presence of CL, but the other females were likely at various stages of proestrus or estrus, which in wolves last an average of 6 and 1 wk, respectively [23]. Cycle stage of the dogs was not known and, because they are not seasonal breeders, cycle stage cannot be estimated by time of year, so the affect of this factor cannot be compared to wolves. Although an explanation for the variability in wolf oocyte yield is not apparent, it is encouraging that females more than 10 y of age still had what, in some cases, were surprisingly high numbers of oocytes (e.g., 73 oocytes retrieved from an 11-y-old female 516).
Assessment of oocyte viability using propidium iodide relied on the ability of healthy, intact plasma membranes in live cells to exclude this dye. This method does not provide any information on the ability of these oocytes to complete nuclear maturation, be fertilized in vitro, or develop into an embryo which could potentially be transferred to a surrogate female where it might initiate and sustain pregnancy. Further in vitro testing of these oocytes must establish these developmental competencies after Cryotop vitrification of dog and wolf oocytes. However, this oocyte vitrification method has led to successful production of off-spring in other species, including humans [10,18,24,25]. Once in vitro maturation, fertilization and embryo culture have been successfully developed for the domestic dog, the ultimate test of oocyte viability post-vitrification, production of young, can be performed. Meanwhile, this technique will allow female wolf gametes to be banked that would otherwise be lost, thus preserving valuable genes that could eventually contribute to the maintenance of higher levels of heterozygosity in future Mexican wolf populations.
Documentation of oocyte viability following vitrification demonstrated that the Cryotop method yielded oocytes that survived vitrification and warming. This was an encouraging result, as it suggested that oocytes from endangered wolves may be vitrified with the Cryotop technique with reasonable success. As additional opportunities occur with aging or spayed females, oocytes may be collected and vitrified for use in future genetic management of captive and even of wild Mexican wolf populations. We anticipate that assisted reproductive technologies for canids will become better established and more efficient in the future, allowing gametes of current and future wolves to be used for optimal genetic management of the species.
In summary, dog oocytes were shown to be a good model for Mexican gray wolves, based on similar percentages of viability following vitrification with the Cryotop technique. These results justified the use of this technique for banking gametes from female Mexican wolves for future use in genetic population management. In addition, these were the first oocytes from either a canid or an endangered species to have been successfully vitrified, based on post-vitrification viability assessment.
Acknowledgements
The authors thank staff at the collaborating institutions for providing tissue samples: Southwest Wildlife Rehabilitation and Education Foundation, Scottsdale, AZ, USA; El Paso Zoo: El Paso, TX, USA; Wild Canid Survival and Research Center, Eureka, MO, USA; New York Wolf Conservation Center, Salem, NY, USA; and we especially thank the Wildlife Science Center, Forest Lake, MN, USA for allowing us to test the protocols first with their generic gray wolves. We also are grateful to the Mexican Wolf SSP and Peter Siminski for travel funds and especially to Drs. M. Kawayama and N. Kagawa for training and assistance in applying the technique they developed. The authors also thank Drs. Speck and Reese and the staff of the Spay and Neuter Clinic of the Animal Protective League of Springfield & Sangamon County, IL, USA for their kind donation of dog ovaries for this project.
References
- Siminski P. Mexican Wolf Hamano S, Koikeda A, Kuwayama M, Nagai T. Full-term development of in vitro-matured, vitrified and fertilized bovine oocytes. Theriogenology 1992;38:1085–90. International Studbook. The Living Desert: Palm Desert, CA, 2008.
- Hedrick PW, Miller PS, Geffen E, Wayne R. Genetic evaluation of the three captive Mexican wolf lineages. Zoo Biol 1997;16: 47– 69.
- Hamano S, Koikeda A, Kuwayama M, Nagai T. Full-term development of in vitro-matured, vitrified and fertilized bovine oocytes. Theriogenology 1992;38:1085–90.
- Hurtt AE, Landim-Alvarenga F, Seidel GE, Jr, Squires EL. Vitrification of immature and mature equine and bovine oocytes in an ethylene glycol, ficoll and sucrose solution using openpulled straws. Theriogenology 2000;54:119 –28.
- Rojas C, Palomo MJ, Albarracin JL, Mogas T. Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryo- biology 2004;49:211–20.
- Merlo B, Iacono E, Regazzini M, Zambelli D. Cat blastocysts produced in vitro from oocytes vitrified using the cryoloop technique and cryopreserved electroejaculated semen. Theriogenology 2008;70:126–30.
- KatayamaKP,StehlikJ,KuwayamaM,KatoO,StehlikE.High survival rate of vitrified human oocytes results in clinical pregnancy. Fert Steril 2003;80:223–24.
- Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 2005a;11:300 – 8.
- Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online 2005b;11:608 –14.
- Homburg R, van der Veen F, Silber SJ. Oocyte vitrification – women’s emancipation set in stone. Fertil Steril 2009;91:1319– 20.
- Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue Reprod Biomed Online 2009; 18:568 –77.
- Vilá´ C, Savolainen P, Maldonado JE, Amorium IR, Rice JE, Honeycutt RL, Crandall KA, Lundeberg J, Wayne RK. Multiple and ancient origins of the domestic dog. Science 1997;276: 1687–9.
- Kutzler MA. Estrus induction and synchronization in canids and felids. Theriogenology 2007;68:354 –74.
- Asa CS, Bauman K, Callahan P, Bauman J, Volkmann DH, Jochle W. GnRH-agonist induction of fertile estrus with either natural mating or artificial insemination, followed by birth of pups in gray wolves (Canis lupus). Theriogenology 2006;66: 1778 – 82.
- Rodrigues B, Rodrigues J. Influence of reproductive status on in vitro oocyte maturation in dogs. Theriogenology 2003;60: 59–66.
- Rodrigues, B, Rodrigues J. Responses of canine oocytes to in vitro maturation and in vitro fertilization outcome. Theriogenology 2006;66:1667–72.
- Wang, X, Catt, S, Pangestu, M, Temple-Smith, P. Live off-spring from vitrified blastocysts derived from fresh and cryopreserved ovarian tissue grafts of adult mice. Reproduction 2009;138:527–35.
- Kuwayama M, Kato O. All round vitrification method for human oocytes and embryos. J Assist Reprod Genet 2000;17:477.
- Lee HS, Yin XJ, Kong,IK. Sensitivity of canine oocytes to low temperature. Theriogenology 2006;66:1468 –70.
- Somfai T, Dinnye=s A, Sage D, Marosa=n M, Carnwath JW, Ozawa M, Kikuchi K, Niemann H. Development to the blastocyst stage of parthenogenetically activated in vitro matured porcine oocytes after solid surface vitrification (SSV). Theriogenology 2006;66:415–22.
- Lopes G, Sousa M, Luvoni, GC, Rocha A. Recovery rate, morphological quality and nuclear maturity of canine cumulusoocyte complexes collected from anestrous or diestrous bitches of different ages. Theriogenology 2007;68:821–25.
- Songsasen, N. and Wildt, D. E. Oocyte biology and challenges in developing in vitro maturation systems in the domestic dog. Anim Reprod Sci 2007;98:2–22.
- Asa CS, Seal US, Plotka ED, Letellier MA, Mech LD. Effect of anosmia on reproduction in male and female wolves (Canis lupus). Behav Neural Biol 1986;46:272– 84.
- Nagy ZP, Chang C-C, Shapiro D et al. Clinical evaluation of the efficiency of oocyte donation program using egg cryo-banking. Fertil Steril 2007;88:S42–S43.
- Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril 2008;89:1657– 64.
See also: