How ovarian transplantation works and how resting follicle recruitment occurs: A Review of Results Reported From One Center
By SHERMAN SILBER
Women’s Health
Download PDF version of Dr. Silber’s Article in Women’s Health Magazine
Summary
Ovarian freezing and transplantation has garnered increasing interest as a potential way of preserving fertility in cancer patients. This special report aims to identify the success rate of frozen compared to fresh ovarian cortex transplantation (in one single series from one center for the sake of consistency), as well as potentially provide insight into the mechanism behind ovarian follicle recruitment [1, 2]. A comparison of fresh versus frozen transplantation techniques is presented, highlighting the similarity and differences between the fresh and frozen transplantation procedures. Much of the literature is scattered case reports with different patient populations and different techniques. This represents an effort to simplify and popularize an approach that has yielded favorable results (all cases recovered ovulation and 75% had successful spontaneous pregnancy) in one single, disciplined study.
Introduction
Ovary freezing and transplantation has garnered increasing interest as a possible way to preserve fertility for cancer patients and even for the decline in fertility, which naturally occurs in all women with aging. Yet these are mostly case reports with no consistent “denominator” to indicate success rates, and no scientific analysis of the mechanism of resting follicle recruitment. The original papers have been published, and this is simply a review of results reported from one center [1-15]. Twenty-two women underwent fresh donor ovary cortex or frozen ovarian cortex transplantation in one center with one surgical technique over a decade of follow up. Eleven were fresh, and eleven were frozen. Recovery in the recipients was measured by return of menses, day 3 FSH, LH, estradiol, AMH, ultrasound, and pregnancy. Patient characteristics are all summarized in Tables 1 and 2 [1].
Table 1: Pregnancy and duration of function of fresh donor ovarian grafts from identical twin sister (9) or non-identical sister allograft (2).
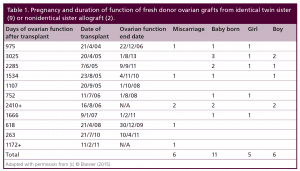
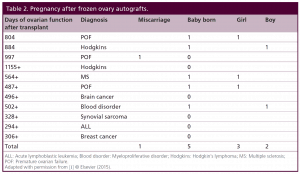
Table 2: Pregnancy after frozen ovary autografts.
All recipients of fresh or frozen ovarian transplants had the same robust return of FSH to pre-menopausal levels by 150 5 days, menstruation by 130 days, and massive AMH elevation by 170 7 days. The AMH then fell to below normal by 240 4 days and remained at that level for many years with normal ovarian cycling and hormonal function. Seventeen babies resulted from 11 fresh and six frozen cycles. Grafts of 1/3 of an ovary cortex lasted as long as 8 years or more despite eventually low AMH. Our results support a hypothesis that ovary freezing and transplantation is clinically a robust method of preserving ovarian function, and it supports a hypothesis that ovarian stromal density gradient can account for the early meiotic arrest of fetal oogonia, and also for “resting” follicle recruitment in the adult.
Successful fresh as well as frozen ovarian cortex transplants in humans were first published in 2004 and 2005, as a case reports, and many other case reports have subsequently followed [1-15]. However there has been no systematic report from one center comparing a large series of both fresh donor and frozen ovary autografts, and there has been little analysis of ovarian function an resting follicle recruitment gleaned from study of these procedures. Nevertheless, there has been a rapidly growing interest in ovarian cortical transplantation despite the absence of a clearly documented success rate for this procedure. The primary impetus for this procedure has been to cryopreserve ovarian tissue prior to sterilizing cancer treatment with the objective of transplanting the tissue back after cancer cure, after the proof of principle was reported in sheep in 1994 [16]. The possibility also loomed of preserving fertility and even hormonal function against the natural decline caused by aging [17, 18].
Oocyte freezing is often mentioned as another alternative for preserving fertility [19, 20, 21]. However, many different centers do it in different ways and most have not verified long-term viability of the oocytes. There could therefore be many disappointed women in years to come. Among the pitfalls to oocyte freezing are too rapid and changing osmolality with dangerously quick osmotic shifts. Also, there is a common failure to ensure a rapid enough freeze, and worse yet not a rapid enough thaw. The commercial kits that are designed to make this “easy” often fail in this regard, and closed freezing is more problematic than open freezing for rapid freeze and thaw. It was not until 2005 that a highly efficient method was published, which stimulated a huge wave of enthusiasm, for this approach, also known as the “bridge” technique (Figure 1 a-f), but it also is very user-dependent, and none of the oocyte vitrification protocols are as fool proof and easy as freezing of ovarian tissue. A benefit of ovary cryopreservation and transplantation over oocyte freezing, however, is the putting off of hormonal menopause as well as preserving fertility. It is well known that unilateral oophorectomy does not diminish fertility, and does not hasten menopause. Thus it is speculatively possible that grafts taken from young women could be used to delay menopause in the future [22, 23, 24].
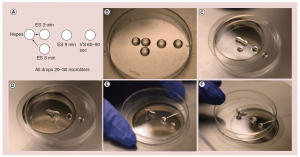
Figure 1: (a) The “bridge” technique for oocyte freezing. (b) Setup for “bridge” equilibration. (c) First “bridge” between ES and isotonic hepes media; three minutes. (d) Second “bridge” equilibration; three minutes (e) Transfer to full concentration ES; nine minutes. (f) Transfer from ES to VS; 60-90 seconds. Caption: High cryoprotectant concentration is not toxic. It is just the rapid osmotic shifts that kill the egg or embryo and give the incorrect impression of toxicity. To avoid over rapid osmotic shifts (that are more poorly tolerated by the egg than the embryo) the original “bridge” technique is best. ES solution droplets are first “bridged” over to the iso-osmotic solution the egg is in, and three minutes later another droplet of ES solution is “bridged” over to the original solution very gradually and continuously raising the osmolality of the solution the oocyte is resting in. ES: Equilibration solution; VS: Vitrification solution.
Ovarian Cortical Transplantation
The technique of ovarian cortical transplantation in this study is not exactly the same as others have reported, [6, 9, 10] but we have already described our technique with great detail in the literature [1, 13, 14]. Except for one case performed with microvascular anastomosis, all were ovarian cortical grafts onto denuded medulla with a classical skin grafting-type technique, the same as was originally reported with our first case [3]. For ovarian tissue freezing, from 1997 until 2008, we used classic “slow freeze,” [3, 25] and since 2008 we have only used “vitrification” [26-28].
Nine of the eleven patients with fresh donor to recipient ovary transplantation were identical twins in whom the donor twin had proven fertility and the other twin had undergone POF. One of the 9 recipients had suffered POF from bone marrow transplantation (BMT) from her identical twin sister, who was now years later, donating her ovary.
More germane to practical clinical application, eleven patients more recently underwent thawing of prior frozen ovarian cortex to restore fertility that was lost from cancer or autoimmune treatment. Eight have long enough follow up to evaluate pregnancy potential (greater than one year). However, one of those eight frozen transplant recipients is intentionally avoiding pregnancy using barrier contraception and is just interested in return of hormonal function.
Results
Since most of the literature is simply composed of case reports of frozen transplants, it was hoped that a single large series of both fresh and frozen transplants from one center with the same techniques and studied physiologically in a uniform way would improve our understanding of resting follicle recruitment, as well as evaluate the clinical robustness of the procedure. Of the eleven menopausal women who underwent fresh transplantation of ovarian cortex from their donor sister, there were eleven healthy babies (5 boys and 6 girls) from 14 pregnancies (4 miscarriages) in 7 of the recipients. Of the 4 out of 11 recipients who did not have a baby, 2 had severe radiation damage to the uterus from BMT, one became pregnant but miscarried and then became menopausal again (her donor sister had a low ovarian reserve in the one ovary which she chose to donate), and the fourth was 40 at the time of transplant. Surrogacy was not recommended in cases like these, because IVF will only yield a small number of eggs, even though they ovulate a good egg each month. It is hard to stimulate those patients and get a large number of good eggs for surrogacy. However, surrogacy would be possible if the patient were to request it, due to her uterine inadequacy. The patients referred here did not want surrogacy. Thus among the recipients of fresh donor ovarian cortex, the majority got pregnant (8/11) and delivered (7/11) 11 live healthy babies. But even the four out of eleven who failed to get pregnant had normal continuing return of hormonal and menstrual ovarian function otherwise. All recipients had return of ovarian function at the same time post-operatively (4.5 months).
Although the clinical results seem promising from this large series of fresh ovarian transplants, the functional results may be even more interesting (Figures 2, 3, 5, 6). Day 3 FSH came down from menopausal range to normal or near normal levels in all eleven cases by 150 days, and menstrual cycling resumed in all eleven recipients by 130 days. Since all developing follicles were destroyed or excluded from the thin (1mm) donor ovarian cortical graft, this would represent the number of days required for resting follicles to be recruited and develop to the ovulatory stage when they would finally become sensitive to cyclic FSH and LH.
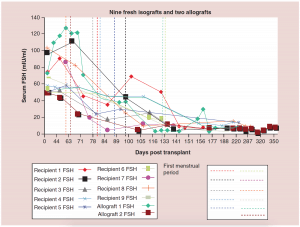
Figure 2: Nine fresh isografts and two allografts.
FSH: Follicle-stimulating hormone.
Adapted with permission from [1] © Elsevier (2015).
The relationship between FSH levels, menstruation, and AMH levels in these fresh transplants is indicative of resting follicle recruitment (i.e. number of functional resting follicles – Figure 2). As the FSH returned to normal by 130 days, the very low AMH level of the recipient began to rise in response to an increasing number of mature gonadotropin sensitive follicles [28-29]. The AMH of the recipient then continued to rise to well above the normal baseline AMH of the donor. Contrary to what might have been expected from earlier studies [30], this indicated no significant loss of follicles from the transplantation, which was also indicated by ultrasonographic examination of stimulated tissues at this period, which showed a very robust follicular response to gonadotropin stimulation when IVF was attempted (third identical twin case). Then by 240 days the AMH of the recipient descended to well below baseline levels, and then remained steady at this level usually for many years (Figure 3) [25-29].
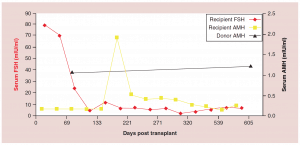
Figure 3: Follicle-stimulating hormone and Anti-Müllerian hormone levels after fresh ovarian tissue.
AMH: Anti-Müllerian hormone; FSH: Follicle-stimulating hormone.
Adapted with permission from [1] © Elsevier (2015).
The auto-transplantation of frozen ovary tissue yielded results almost identical to fresh (as might have been guessed by failure to notice any histological damage from freezing) [25-28]. As with fresh ovary transplants, the FSH always returned to normal in these cases by 150 days without exception, and menstrual cycles resumed shortly before that. The return to normal was no different for slow freeze cases than for vitrification cases. In all of these cases, just like with fresh transplants, the AMH rose to very high levels shortly after the FSH returned to normal, at about 170 days. Then the AMH, exactly as with fresh transplant cases, came down to a lower baseline level by 240 days and remained at that lower indefinitely, although our earliest frozen cases were less than one to four years ago (Figure 4, 5a-c).
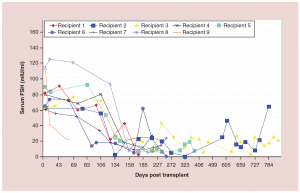
Figure 4:Follicle-stimulating hormone curves for frozen ovary transplants
FSH: Follicle-stimulating hormone.
Adapted with permission from [1] © Elsevier (2015).
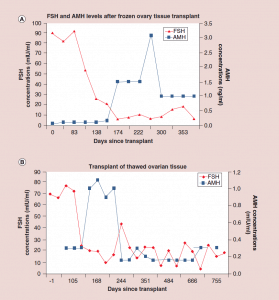
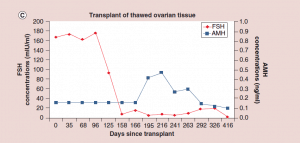
Figure 5: Follicle-stimulating hormone and Anti-Müllerian hormone levels after frozen ovary tissue transplant.
(a-c) FSH and AMH levels for individual patients after frozen ovary tissue transplants.
AMH: Anti-Müllerian hormone; FSH: Follicle-stimulating hormone. Adapted with permission from [1] © Elsevier (2015).
After their transplants, eight of the eleven frozen cases have regained ovarian function for over one year, and thus, those cases are suitable to look at pregnancy potential. However, one of these eight has chosen not to get pregnant yet and is using barrier contraception. Of the remaining seven, six have gotten pregnant although one miscarried. Thus frozen results were similar to fresh in terms of hormonal function and pregnancy outcome. Quite remarkable was the repeatable concordance of functional results thus far seen in all 22 cases of fresh ovary donor transplantation and frozen ovary autotransplantation.
Discussion
What began as one usual case of discordance between identical twins for idiopathic POF, which was treated successfully with ovarian cortex transplantation, eventually led to a large series of 22 human ovarian transplants, 11 fresh from donor sisters, and 11 with ovarian cortical tissue that was frozen for preservation of fertility prior to treatment for cancer or autoimmune disease. The success in 11 cases of frozen as well as the eleven cases of fresh ovary transplants have vast implications for cancer patients. In all of these frozen cases just like fresh, there is a robust return of menstrual cycling and normal ovarian function at precisely the same time post-operatively (4.5 months) in every single recipient, and five healthy babies out of seven recipients of frozen tissue who were trying to get pregnant, not to mention the 11 babies from 11 fresh transplants. Thus, the pregnancy and live baby rate for fresh as well as for frozen ovary transplantation is about 75%. There are many case reports of healthy babies delivered to otherwise sterile cancer survivors via frozen ovary transplants, which supports the enthusiasm for this approach, despite being only scattered case reports [7, 8, 10, 11, 12, 15, 17].
But there may be an additional scientific value to these observations i.e. understanding resting follicle recruitment and fetal oocyte meiotic arrest. Firstly, this was an unusual opportunity in humans to compare fresh ovarian tissue transplantation to frozen, in one center using the same uniform technique, and there was no difference in an unusual opportunity in humans to compare fresh ovarian tissue transplantation to frozen, in one center using the same uniform technique, and there was no difference between fresh and frozen. But aside from that clinical benefit, data was obtainable which may afford a better understanding of “resting follicle” recruitment in the human. Primordial follicles are not subject to hormonal stimulation. Just how they are recruited to develop, and how quickly, into secondary and antral follicles that are sensitive to hormonal stimulation, is a puzzle worth solving, as is the puzzle of early fetal oocyte meiotic arrest.
In fact primordial follicles should not be referred to as “resting” follicles, because in truth they are “arrested” in early fetal life at meiotic prophase. When they are recruited in adult life to develop into secondary follicles and eventually ovulatory follicles, in fact they are escaping from their “arrested” state. In early fetal life, primordial germ cells (PGC’s) meet retinoic acid at the genital ridge, which upregulates the Stra8 gene that stimulates the primordial germ cells in females to enter meiosis [31-34]. In males, the Sertoli cells of the testis block this effect of retinoic acid, and so in the male fetus, the germ cells do not enter meiosis. Why do these fetal oogonia not just continue through meiosis, and eventually all rapidly disappear by the time of birth? Why don’t they just keep on developing into eggs and therefore eventually be gone by the time of birth? Why are they “arrested” in early meiosis? And could that also be the mechanism that controls recruitment of resting follicles in the adult?
While the female germ cells invade the very dense stroma of the tunica albuginea, which becomes the ovarian cortex, the tunica albuginea of the testes really is no different histologically from the ovarian cortex except for the absence of eggs. In the mouse, the time period around meiotic initiation (exactly when Stra8 is upregulated) is the key period for setting up growing follicles in the medulla and non-growing follicles in the cortex, which supports our clinical observations of too many follicles beginning to grow and develop if they do not invade the cortex [32, 34].
There is a gradient of stromal density in the ovarian cortex with the most tissue pressure and density near the surface, and gradually getting less dense toward the center and finally the least dense tissue is the ovarian medulla (Figure 6) [34]. It has already been postulated via in vitro follicle culture that pressure may have a huge effect on resting follicle development [35, 36]. Also it is well known that the “resting” (or arrested is a better term) primordial follicles are recruited first from the least dense internal stroma of the cortex and develop toward the medulla, which is the least dense tissue and hence the least pressure (Figure 6). The stromal density gets less and less the further inward from the surface of the ovarian cortex. Then once the follicles become Graafian follicles and ready to ovulate they are not on the ovarian surface, but just burst through the surface from the medulla to ovulate (Figure 6).
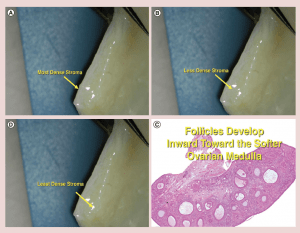
Figure 6: (a-c) Stromal density gradient in ovarian cortex.
(d) Follicles develop inward toward the softer ovarian medulla
The results in this series of ovarian transplants support the theory that it is tissue pressure or stromal density which governs resting follicle recruitment, and which allows the fetal ovarian cortex to arrest the oogonia in early meiosis [35, 36]. Roughly not too far off from Gougeon’s predictions, [37] in our 22 cases of ovarian transplant, whether fresh or frozen, menstrual cycling returned at 130 days, day 3 FSH returned to normal at 150 days, AMH levels rose at 170 days to very high above normal levels, and then went down to below normal levels at 240 days. This indicates that shortly after the cortical graft was placed a massive recruitment of “resting” follicles occurred and depleted a large number of eggs from the graft. Then after full return of the normal tissue pressure, the AMH stabilized and a normal rate of recruitment resumed.
Thus both the embryologic data on fetal meiotic arrest of oogonia that were stimulated to enter meiosis by the presence of retinoic acid, and arrested by the ovarian cortex (tunica albuginea in the male) and our results with ovarian transplantation, support the unifying hypothesis that fetal meiotic arrest and adult “resting” follicle recruitment is just an inexorable process governed by cortical tissue pressure. It should be readily understood that it is not just oogonial meiosis that is arrested by the fetal ovarian cortex, but also non-meiotic resting oocyte development, since meiosis and pre-antral recruitment and development are separate phenomena.
Future Perspective
Firstly, ovarian freezing and transplantation should no longer be considered experimental moving forward. Secondly, this could provide a unifying theory for adult recruitment and fetal arrest of primordial follicles. Without a steady controlled release of “resting” follicles in the adult, women would run out of eggs prematurely. Similarly, if fetal oocyte arrest did not occur after meiotic activation, there would be no oocytes remaining at birth. Similarly, if there were not a slow, steady, monthly release of primordial (“resting” or better, “arrested”) follicles from the resting phase, without any hormonal regulation, related to tissue pressure gradients in the ovarian cortex (the same tissue as the dense stroma in the tunica albuginea of the male), all the adult follicles also would soon be gone. Thus we propose a unifying theory for both fetal oocyte arrest and adult resting follicle recruitment.
Executive Summary
Introduction
- Ovary freezing and transplantation is gaining interest as a means of fertility preservation.
- Until now, no systematic report from one center comparing a large series of both fresh donor and frozen ovary autographs has been published.
Ovarian Cortical Transplantation
- For ovarian tissue freezing from 1997 through 2008, classic “slow freeze” was used and since 2008 only “vitrification” has been utilized.
- Nine of the eleven patients with fresh donor to recipient ovary transplantation were identical twins in whom one had proven fertility and the other had experienced POF.
- The additional eleven patients received thawed tissue from previously frozen ovarian cortex to restore fertility that was lost due to cancer or autoimmune treatment.
Results
- Of the eleven menopausal women who underwent fresh transplantation of ovarian cortex from their donor sister there were eleven healthy babies (5 boys and 6 girls) from 14 pregnancies (4 miscarriages) in 7 of the recipients.
- Seven of the eight women that received a frozen transplantation and were suitable for follow up, six conceived (including one miscarriage).
- All 22 cases experienced robust return of normal FSH by 150 days, menstruation by 130 days, and massive AMD elevation by 170 days.
Discussion
- Results of this study indicate equivalent success rates in fresh versus frozen ovarian transplantation.
- Additional scientific value stems from these observations in that make a greater understanding of resting follicle recruitment and fetal oocyte meiotic arrest possible.
- The results of this ovarian transplantation series support the theory that tissue pressure or stromal density governs resting follicle recruitment, which allows the fetal ovarian cortex to arrest the oogonia in early meiosis.
Future Perspective
- Studies of this nature should allow for ovarian tissue freeze and transplantation to no longer be regarded as experimental.
- The results of this study lend themselves to future investigation of the unifying theory of resting follicle recruitment and fetal meiotic arrest.
Download PDF version of this article
Bibliography
- Silber SJ, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod. Biomed. Online. http://dx.doi.org/10.1016/j.rbmo.2015.02.010.
- Silber S. Unifying theory of adult resting follicle recruitment and fetal oocyte arrest. Reprod. Biomed. Online. http://dx.doi.org/10.1016/j.rbmo.2015.06.022.
- Silber SJ, Lenahan KM, Levine DJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N. Engl. J. Med. 353, 58- 63 (2005).
- Silber S. Ovary cryopreservation and transplantation and transplantation for fertility preservation. Mol. Hum. Reprod. 18(2), 59-67, 2012.
- Demeestere I, Simon P, Englert Y, Delbaere A. Preliminary experience of ovarian tissue cryopreservation procedure: alternatives, perspectives and feasibility. Reprod. Biomed. Online. 7, 572-579 (2003).
- Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum. Reprod. 25, 1590-1591 (2010).
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 364, 1405-1410 (2004).
- Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of preimplantation. Fertil. Steril. 99, 1503-1513 (2013).
- Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann. Med. 43, 437-450 (2011).
- Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N. Engl. J. Med. 353, 318-321 (2005).
- Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 379, 588 (2012).
- Sanchez M, Alama P, Gadea B, Soares SR, Simon C, Pellicer A. Fresh human orthotopic ovarian cortex transplantation: long-term results. Human. Reprod. 22, 786-791 (2007).
- Silber SJ, DeRosa M, Pineda J, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Human. Reprod. 23, 1531-1537 (2008).
- Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N. Engl. J. Med. 356, 1382-1384 (2007).
- Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N. Engl. J. Med. 359, 2617-2618 (2008).
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum. Reprod. 9, 597-603 (1994).
- Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod. Biomed. Online. 25, 128- 32 (2012).
- Stoop D, Cobo A, Silber SJ. Fertility preservation for age related fertility decline. Lancet. 384, 1311-1319 (2014).
- Homburg R, van der Veen F, Silber SJ. Oocyte vitrification–women’s emancipation set in stone. Fertil Steril. 91, 1319-1320 (2009).
- Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G. The efficacy and safety of human oocyte vitrification. Semin Reprod Med. 27, 450-455 (2009).
- Cobo A, Domingo J, Perez S, Crespo J, Remohi J, Pellicer A. Vitrification: an effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 10, 268-273 (2008).
- Lass A, Paul M, Margara R, Winston RM. Women with one ovary have decreased response to GnRHa/HMG ovulation protocol in IVF but the same pregnancy rate as women with two ovaries. Hum Reprod.12, 298-300 (1997).
- Zhai A, Axt J, Hamilton EC, Koehler E, Lovvorn HN, 3rd. Assessing gonadal function after childhood ovarian surgery. J Pediatr Surg. 47, 1272-1279 (2012).
- Gosden RG, Telfer E, Faddy MJ, Brook DJ. Ovarian cyclicity and follicular recruitment in unilaterally ovariectomized mice. J Reprod Fertil. 87, 257-264 (1989).
- Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 11, 1487-1491 (1996).
- Keros V, Xella S, Hultenby K, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 24, 1670-1683 (2009).
- Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 18, 568-577 (2009).
- Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil. Steril. 94, 2191-2196 (2010).
- La Marca A, Giulini S, Tirelli A, et al. Anti-Mullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum. Reprod. 22, 766-771 (2007).
- Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 140, 462-471 (1999).
- Anderson EL, Baltus AE, Roepers-Gajadien HL, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA. 105, 14976-14980 (2008).
- Byskov AG, Guoliang X, Andersen CY. The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol. Hum. Reprod.. 3, 795-800 (1997).
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA. 103, 2474-2479 (2006).
- Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Dev. Dyn. 194, 177-192 (1992).
- Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomater. 32, 2524-2531 (2011).
- Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J. Assist. Reprod. Genet. 28, 3-6 (2011).
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum. Reprod. 1, 81-87 (1986).