
Many of our pioneering advances in infertility treatment and preserving human infertility for our patients, also have been used by us in helping zoo and wildlife officials save endangered (and even near extinct) species. We have performed microsurgery to restore or preserve fertility in chimpanzees, gorillas, South American bush dogs, Mexican wolves, orangutans, and Mongolian wild horses. We have consulted at the National Zoo in Washington, D.C., the San Diego Zoo, the St. Louis Zoo, the Pittsburgh Zoo, the Tulsa Zoo, and the Amsterdam Zoo to help them in their efforts to save endangered species.
We have frozen ovaries in animals that are destined to die off for later ovary transplantation back to related species to be able to increase their population. We have even solved the seemingly intractable problem of freezing fish and frog eggs of species that are almost extinct. We have performed IVF and gestational surrogacy on orangutans to improve to ability of hand raised females to rear their own baby orangutans.

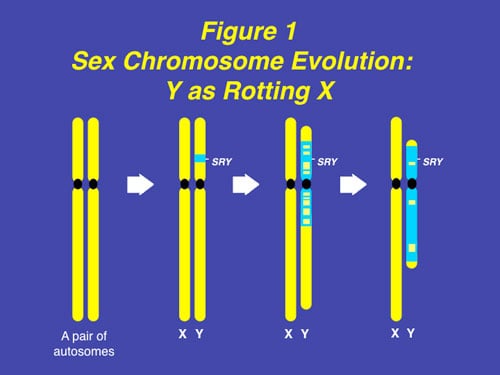
We have collaborated with paleontologists to try to understand why the dinosaurs went extinct, and thereby learn how to save species which are at similar risk today. For example, in our elucidating the genes on the Y chromosome which control male infertility, we deciphered the whole evolutionary history of the X+Y chromosomes, and in fact in general the evolutionary history of sex determining chromosomes in general. We postulated that sex determining chromosomes like the Y are so unstable that fertility genes are often compromised in animals which evolved sex determining chromosomes.
So why would sex chromosomes like X+Y evolve in the first place if they negatively impact fertility? The reason is that they assure a balanced sex ratio of offspring 50:50. Otherwise, if temperature alone determined the gender of offspring, in periods of massive global warming, or cooling, the birth of a skewed sex ratio, like all males, would result in extinction.
Thus although our primary goal is improving fertility treatment for our patients our discoveries also help save endangered species from disappearing from the globe.
Video Documentaries
Research Papers
Understanding Extinction: Human Male Infertility and the Dinosaur
We were the first to study the molecular genetics of human male infertility and the Y chromosome (beginning in 1992) which helped to elucidate the evolution of X and Y chromosomes (Figure 1). Our further study of the Y chromosome and sex determining chromosomes in other species then surprisingly helped to clarify a likely mechanism for dinosaur extinction, the biggest question all of us have entertained from our earliest childhood days.
There have been many claims in the popular press of “discoveries” on how the dinosaurs went extinct. These claims all relate to climate change events that occurred 65 million years ago, a fact which no one disputes did occur. But none have explored the biology of how so many animals escaped extinction while the dinosaurs and at least half of all other species did not. For example, why did large dinosaurs, as well as small dinosaurs the same size as chickens go extinct, but birds survived? Possibly the evolution of sex chromosomes (like the Y) holds the answer to this question.
Our studies of the Y chromosome and male infertility suggest that the default mechanism throughout the animal kingdom for determining the sex of offspring is the temperature of egg incubation, and that genetic sex determination (based on sex chromosomes like X and Y) has evolved many times over and over again in different ways, in different genera throughout biological history, as a more foolproof method than temperature variation for assuring a balanced sex ratio in offspring. The absence of such a genetic sex determining mechanism in dinosaurs may have led to a male-skewed sex ratio when global temperature dramatically changed 65,000,000 years ago, resulting in a preponderance of males, and consequentially a rapid decline in the dinosaur population.
Technical Introduction to Concept of Extinction Based on Massive Global Climate Change
Genetically based sex determination mechanisms have evolved independently in many different genera numerous times over throughout the history of life on earth to ensure a balanced male-female sex ratio. Mammals, birds, all snakes and most lizards, amphibians, flying insects, worms, and some fish, employ specific “sex-determining” chromosomes or genes to determine the sex of the embryo. This mechanism is termed GSD, for “genetic sex determination.” In mammals, GSD is controlled by the SRY gene on the smaller Y chromosome of the male. Contrastingly, in all birds, fowl, and snakes, who also use GSD, the smaller sex chromosome results in a female rather than a male offspring.
However, not all animals employ GSD for sex determination and in fact, GSD is not the original default mechanism for sex determination. Crocodiles and alligators, turtles, some lizards, and many fish employ environmental, or temperature-dependent sex determination (TSD) of offspring. This means that there is no genetic predetermination of gender. In these animals, it is merely the temperature at which the early embryos are incubated that determines whether the primitive gonad will differentiate into a testis or an ovary, and therefore whether the embryo will become a boy or a girl.
Thus, temperature dependent sex determination (TSD) is thought to be the primordial mechanism that triggers either testicular or ovarian development in the early embryo. The development of either a testis or an ovary from the early embryonic primitive gonad is actually a result of a huge multitude of genes. The sex-determining gene (like SRY on the Y chromosome in we mammals) is simply a trigger to activate all those downstream cascades of many other genes elsewhere in the genome that actually do orchestrate testis or ovarian development. The Y chromosome is just a trigger to activate all these other testis making genes that would otherwise be activated by temperature.
The various modes of genetic sex determination (GSD) like our Y chromosome have evolved many times over and over again to override TSD, completely independently and very differently, in the whole history of life on earth. There is no similarity of the Y chromosome, or the sex-determining “trigger” gene, in mammals, lizards, frogs, fruit flies, or worms, except that the male determining chromosome (by definition the Y) is smaller than its counterpart (the X). In snakes and birds, the female determining trigger gene (the W by definition) is smaller than its counterpart (the Z), but they are completely different from each other in all genera.
Evolution, therefore, appears to favor the eventual repeated appearance over and over again in different ways of genetic based sex determination (GSD), we postulate, because animals not using this mechanism are at risk of extinction due to a skewed sex ratio that could result from massive global environmental temperature change. Previously we have shown mathematically that if there are several generations of a preponderance of male offspring, the species cannot survive. This recurring hazard favors the repeated evolution of a sex determining “trigger” gene in all genera (to protect against the disastrous effect of environmental vicissitudes on the sex ratio of offspring).
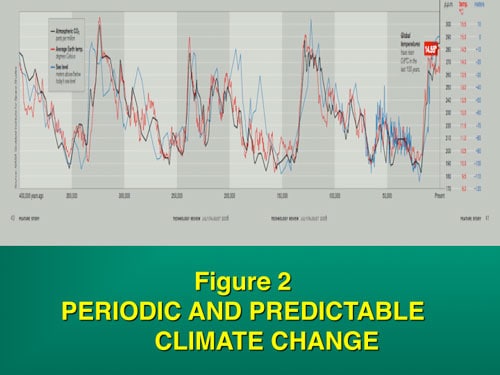
Global temperature spikes predictably occur every 100,000 years, and to a lesser extent in harmonics of every 25,000 years(Figure 2). A massive global temperature change can skew the sex ratio of TSD offspring and we have postulated that this played a role in the demise of the dinosaurs. Global warming today, which far exceeds the usual 100,000-year spike, similarly represents a current risk for extant TSD species. This theory originally suggested in 1989, was based on paleontologic evidence, before we understood the unusual sequence of the Y chromosome and in turn all sex determining chromosomes and the evolution of sex chromosomes. But now our better understanding of the DNA sequence of sex chromosomes, and of the survival of avian dinosaurs, gives even stronger credence to such a view.
Disadvantage of GSD
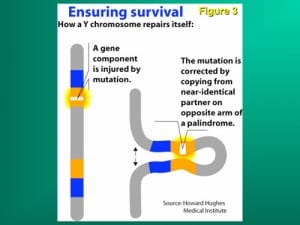
Despite the survival advantage of genetic sex determination, there is, however, a survival disadvantage. The disadvantage of an evolving sex determining chromosome (which guarantees a balanced sex ratio) is its eventual decay caused by the absence of meiotic recombination during gametogenesis, and in humans the subsequent loss of spermatogenesis genes, causing male infertility. This mechanism has been confirmed in studies of infertile males, in association with the full sequencing of the Y chromosome. Hence, despite the protection that GSD affords against catastrophic global temperature shifts, and its necessity for the evolution of endothermic mammals, male infertility is increased because of the accumulation on the Y chromosome of a dense concentration of spermatogenesis genes in a region that is chromosomally unstable because of failure of chromosomal recombination during meiosis (Figures 1 and 3).

Similarly a ZZ-ZW system may have protected birds from the global K-T extinction of their cousins, the dinosaurs and flying pterosaurs. However, female birds only have one working ovary on the left side, and if it is removed, the remaining right-sided vestigial gonad becomes a testis and so the hen, for example, would become a rooster. Hence, the ZZ-ZW female bird may have avoided global extinction by employing a GSD based mechanism for sex determination, but may have paid a price for this by having only one ovary. The human male’s price for this GSD security is declining sperm count.
Thus, unexpectedly, through our efforts to understand the molecular genetics of infertility, we have stumbled upon an explanation for the deepest mystery of our childhood, and our biggest fear, extinction (Figure 4).
Sex Determination and Extinction
Gender differentiation into male or female is accomplished either by variation in the temperature at which eggs are incubated like crocodiles and alligators and turtles, or by genetic mechanisms (Figure 5). Efficient sexual reproduction in most species requires a balanced population of males and females. Mammals universally give rise to a roughly equal number of male and female offspring by relying on the Y chromosome’s testis-determining gene, SRY, to trigger testis development. However, the SRY gene is not found in any other genera that use GSD (e.g. birds, insects, amphibians, lizards, snakes, etc.) and indeed, genetic modes of sex determination (GSD) in unrelated animals have arisen differently and independently many times over.
Despite highly varied “triggering” mechanisms in a variety of vertebrates, most animals share the same or similar, highly conserved downstream genes that control testis or ovary development and hence sex differentiation. The genes that cause the primitive embryonic gonad to become a testis or an ovary are virtually the same for all animals on the planet. Thus, the downstream genetic programs involved in both GSD and environmental modes of sex determination (TSD) are very closely related, almost the same in all animals. This is perhaps not unexpected, given that the development of the testis and ovary and their constituent cells is broadly similar in amphibians, reptiles, mammals, and birds. However, the trigger mechanisms, whether GSD or TSD, which direct those downstream genes to produce testis or ovary development from the primordial indifferent gonads are quite different. TSD animals rely on a potentially precarious relationship with their environment where a balanced sex-ratio is dependent on the temperature of egg incubation (Figure 5). Nonetheless, these two sex-determining mechanisms, whether a sex-determining gene or random variation in temperature of egg incubation, all have the same common goal of assuring a balanced male-female sex ratio.
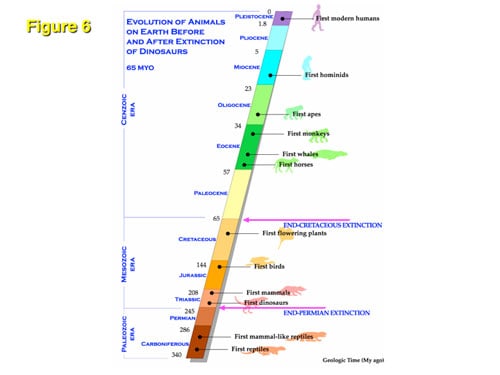
While the precise environmental effects of the great impact event of 65 million years ago are open to debate, it is not contested that profound global environmental changes occurred that led to the extinction of dinosaurs. Yet this global “K-T” event 65 million years ago, when the dinosaurs went extinct, did not cause the mass extinction of mammals, birds, fruitflies, frogs, or snakes (Figure 6). We suggest the reason for this could be that changes in global temperature would have had a more pronounced extinction effect on TSD-dependent animals than on animals using GSD. We will answer at the end of this paper why alligators, crocodiles, and turtles (which are also TSD animals) survived the K-T event even though dinosaurs and pterodactyls did not.

Relationship Between Dinosaurs and Their Relatives
Dinosaurs and crocodiles are members of the Archosauria, a major group of diaspsids that appeared in the Early Triassic, some 245 million years ago. By the Late Triassic (208 million years ago) the dominant representatives of Archosauria were dinosaurs, champosaurs, pterosaurs, and crocodilians. Modern birds were then derived from avian archosaurs that first appeared during the Jurassic (about 170 million years ago) and expanded their range in the Cretaceous period where they would have shared the skies with the dominant pterosaurs which flew but were not birds, (pterodactyls). Crocodilians (TSD-dependent) and birds (GSD-dependent) are the only Archosaurian taxa that have persisted to this day, both very close relatives of the dinosaur. We propose that avians (birds) survived because of their GSD system for gender determination. Crocodilians and other TSD species (but not dinosaurs) survived either because they could adapt successfully to the changing environment or because the global temperature change skewed their sex ratio toward predominantly female, which ironically could have been advantageous rather than disadvantageous to their survival.
Studies on oviparity in the American alligator demonstrate a close physiological link between crocodilian and ancient archosaurian reproductive function. In common with birds, this species has separate uterine regions for formation of the egg membranes and calcareous layers, indicating that extinct reptiles shared a common mode of egg production with contemporary archosaurs. Furthermore, based on skeletal comparisons, fossil evidence suggests that like modern crocodilians, dinosaur hatchlings had relatively mature perinatal pelvic girdles, an observation that is consistent with their sharing the same mobility as modern crocodile young. Furthermore, recent applications of spiral computerized tomography to fossil endocasts has provided compelling evidence that the Allosaur brain case was organized along similar lines to modern crocodilians and quite distinct from that of birds. These comparisons are significant because they suggest physiological similarities between crocodilians and extinct dinosaurs. Birds developed GSD in parallel with their endothermy some 170 million years ago, whereas dinosaurs more likely employed TSD for sex determination.
Molecular Evolution of Sex Determining Chromosomes
The inherent stability of GSD in mammals and birds ironically is due to the subsequent atrophy of the sex-determining chromosome, e.g. the human Y. The driving force for this atrophy process is selective failure of meiotic recombination between the sex chromosomes, leading over time, to the gradual degradation of the non-recombining portion of the sex-determining chromosome (Figure 1). This decay of what was previously an ordinary paired autosome has resulted in selective hyperexpression and inactivation of its paired mate (X-inactivation), bringing parity of gene dosage expression between males and females.
ZZ-ZW birds and some advanced snakes have highly heteromorphic sex chromosomes with extensively atrophied W chromosomes like the atrophied Y in mammals and in fruit flies. However, many snakes, and all amphibians, have homomorphic sex chromosomes, which means that they are virtually indistinguishable in size from each other. In many of these species with GSD sex chromosomes that are homomorphic, an environmentally-induced interchangeability of sex determining mechanisms is occasionally observed. That is, temperature can still override the genetic sex determination system. No GSD species with heteromorphic sex chromosomes, however, is known to display this phenomenon, and evolutionary drive is consistent with GSD eventually dominating TSD due to its irreversibility once the sex-determining chromosome has atrophied. Once atrophy of the sex-determining chromosome has commenced with compensatory hyperexpression of its mate to provide gene dosage parity, there is no going back to TSD.
Evolutionary Accumulation of Testis Specific Genes to the Y and the Subsequent Threat to Male Fertility
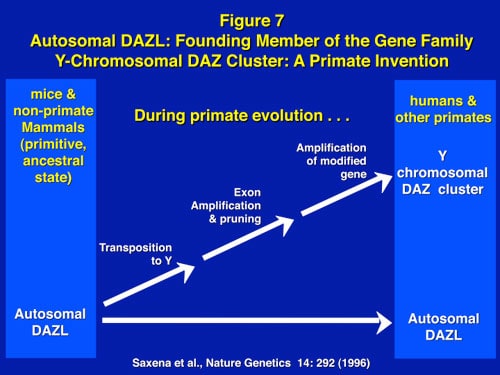
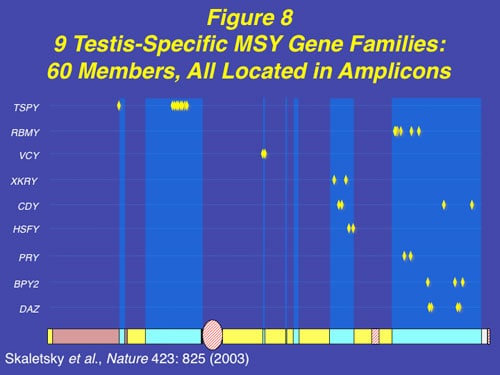
Along with the decay of most of the ancestral autosomal genes on the Y chromosomes, there is a parallel accumulation of other genes on the Y that control spermatogenesis. This inevitably occurs because the region next to the testis-determining gene, which does not recombine during meiosis, is a “safe harbor” for genes which are beneficial to the male but detrimental to the female (Figure 7). “Sexually antagonistic” male benefit genes, which are testis-specific and therefore, enhance male fertility, but would be detrimental to female fertility, have thus been accumulated and amplified with generation of multiple copies and repeat sequences on the non-recombining region of the Y over the course of 300 million years (Figure 8). Thus, a functionally coherent concentration of testis-specific genes in multiple copies has arisen on a labile Y chromosome that is subject to deletions and inversions caused by massive direct and inverted regions of nucleotide identity (amplicons and palindromes), and is a significant cause of human male infertility. There is a fragile balance between “gene conversion” with chromosome repair by recombination between the palindrome arms, and their frequent deletion due to a mistaken homologous recombination between massive ampliconic repeat sequences on the Y. Thus, the inevitable evolution over and over again of sex-determining chromosomes must be very important for species survival, since it also can result in a major threat to male fertility in animals with a Y chromosome.
If you have any questions, you may call us at (314) 576-1400.





















