Dr. Silber Explains
Egg Freezing to Preserve Your Fertility For When You Are Older
We were the first to introduce successful egg freezing in the United States with vitrification, so we have the longest experience with it in America. We are a full-service high-quality fertility clinic located in what has consistently been rated as one of the very best hospitals in America. Our facilities are beautiful and very modern and located in a spectacularly elegant, all green, non-urban location with rolling hills, large estate homes, lakes, and clean fresh air. We are a serious long-standing well-established medical center, NOT a fly-by-night “egg-freezing” small start-up company that is here today and will be gone, or sold, tomorrow.
We are actually much less expensive than the start-up “used car” like companies that promote discounts and encourage gullible patients to “share” eggs and cost. We do not try to suck people in by holding “egg freezing parties” with wine, cheese, and music. We are not in the Cayman Islands or the Bahamas or in the congested big cities of the east and west coasts, with start and stop heavy traffic with honking horns and impersonal roughness.
We are not just an “egg business” cropping up quickly by venture capitalists – planning to show a quick profit and then sell for a huge capital gain for their investors. We are a serious and established properly-regulated professional medical center known for its brilliance and kindness. We have no “investors” to answer to with profit targets. We are not going away tomorrow.
That said, let’s explain in detail the science of your biological clock, the technology of egg and embryo freezing, and clinically how we can stop your biological clock so that you can put off child-bearing until you are ready. Of course, we don’t want to mislead you into casually thinking that freezing your eggs is 100% or a guarantee. You need thoughtful non-remunerative oriented physician counseling to make sure you fully understand what this is all about.
For those that are so wealthy, they can afford any price, let us warn you that you cannot judge the quality of medical service by the price tag. We are very moderately priced. You can only judge the quality of the medical service by the character and quality of the doctors.
I have written that it is easier to get pregnant when you’re younger than when you’re older, and so we have developed a wonderful new method for getting pregnant with your own eggs, even at an older age.
Everyone wants their child to have their DNA, not a stranger’s. That is why we have become so good at helping older women and women with very few eggs get pregnant with their own eggs. But in some cases, the woman is simply out of eggs completely. When that is the case, her frozen eggs from when she was young are quite successful.
It Is The Age Of The Eggs And Not The Age Of The Woman That Matters
Much older women (late forties and fifties) have no difficulty getting pregnant so long as the eggs are from when she was young. The age of the uterus is not what is significant in the high pregnancy rate of these patients, but rather the fact that the eggs came from when she was younger. The main determinant of pregnancy rate is the age of the eggs, not the age of the woman. Women as old as sixty-three years of age have gotten pregnant quite easily with young eggs and have delivered healthy, happy babies.
You Can Save Your Eggs for Later: Egg Freezing & Banking
There are many women in their twenties and early thirties who just don’t want children yet. But they are afraid to put off having children into their mid- to late- thirties, or early forties, for two reasons: (1) They are afraid that with their biological clock ticking, they will not be able to have children if they wait another ten years, and (2) they are afraid that if they do get pregnant later they will be in an age category where this poses a high risk of abnormal embryos and chromosomal defects in their children. These women can undergo IVF while they are still young and have their eggs successfully frozen and stored for later. At a later date, the eggs can be thawed and fertilized with their partner’s sperm, she can get pregnant, even when she is older, and with no increased risk of Down syndrome.
Although human embryos can be successfully frozen and thawed and can result in happy, healthy babies until very recently, eggs could not be successfully frozen and thawed. The success rate in freezing eggs had always been extremely low, but this has all changed now with the vitrification method, which we were the first to introduce in the United State. We can simply save these young frozen eggs for now, potentially for subsequent IVF when the woman is older.
Both egg and ovary freezing are now available for women who just feel a need to delay childbearing until their mid- to late- thirties and forties, by which time their egg supply will very likely have been depleted. These are wonderful options for the woman who wants her own genetic child but does not anticipate starting a family for many years.
Freezing embryos for a future date does not solve the problem for unmarried women who want to have children in the future but have not yet met the right man. For these women, freezing their eggs, or even an entire ovary, would be the ideal solution. Until very recently, this holy grail of IVF was unattainable. The reason is that in order for fertilizable eggs to be retrieved, they must be in a mature state of development, with a complex alignment of chromosomes, and this makes them susceptible to even the slightest ice-crystal damage. However, with the new technique of vitrification, first introduced by us in the United States, ice-crystal formation is avoided completely, and the results indicate that very high pregnancy rates can now be achieved with frozen eggs. Thus, a woman who knows that she is nearing a time when she will lose her fertility because she is nearing her 30’s or late 30’s, because of her biologic clock, can now freeze her eggs when she is young, or even a piece of one ovary, and have her babies later.
This new technique of freezing, called “vitrification,” avoids the damage caused by ice forming inside the cell. With vitrification, you are not trying to pull every last molecule of water out, because it is impossible to do this 100%. In fact, 70% of the cell is water, and at best you can reduce that to 30%. So with the conventional controlled rate slow-freezing technique, there is always going to be some intracellular ice crystal formation, causing some damage to embryos, and severely damaging most eggs. Vitrification, on the other hand, uses a super high concentration of antifreeze (DMSO and ethylene glycol), and drops the temperature so rapidly that the water inside the cell never becomes ice. It just instantaneously super-cools into a solid with no ice crystal formation at all. Thus, there is no damage at all to the frozen (“vitrified”) egg.
We can now freeze and thaw, and even refreeze and rethaw, with impunity, using this new protocol. With conventional “slow freezing,” the temperature of the embryo goes down at precisely 0.3º C per minute. With vitrification (using four times the concentration of antifreeze, or cryoprotectant), the temperature is dropped at 23,000º C per minute, i.e., 70,000 times faster. At that speed of cooling, and at that concentration of antifreeze, ice crystals simply cannot form.
Of course, it is not quite as simple as it might sound. Such high concentrations of antifreeze, in a few minutes, could cause osmotic damage to cells. Therefore, the embryos (or eggs) must first be placed in lower concentrations of antifreeze (and sucrose to draw some water out), and then into higher concentrations before instantaneous freezing. Then when the time comes to thaw the embryo, it must be instantaneously warmed, immediately taken out of the high concentration of antifreeze, and then placed into a solution with a lower concentration, in order to avoid osmotic toxicity. This requires more skill than conventional freezing, but it is faster, cheaper, and most importantly, avoids all freezing damage to either eggs or embryos. Such a reliable method of embryo freezing gives the IVF program much greater ability to avoid dangerous multiple pregnancy and makes scheduling for procedures much simpler for the patient. Our frozen embryo pregnancy rates are virtually 100% with this technique and we can freeze embryos without hurting at all your chance of conception. But more spectacularly, we can now confidently freeze eggs this way also.
Using this vitrification technique for freezing, we can preserve eggs as well as embryos and sperm. This allows us to preserve the fertility of young women for the future in egg banks if they wish to delay childbearing. In St. Louis, we have demonstrated for the first time that an entire ovary can be removed and then grafted back after freezing and thawing so that even a menopausal woman can gain back her youthful fertility many years later. This new capability will be especially important to women undergoing treatment for cancer because all the eggs that might have been lost to chemotherapy can be preserved by first removing, freezing, and storing her ovary for use later. However, most healthy women will just prefer the less-invasive freezing of individual eggs. In order for this to give her a degree of assurance, we must use our minimal stimulation protocol that gives the highest pregnancy rate per egg, and no side effects or complications.
How the Biological Clock Works and Why You Should Freeze Your Eggs.
The Science of Why You Become Infertile As You Get Older
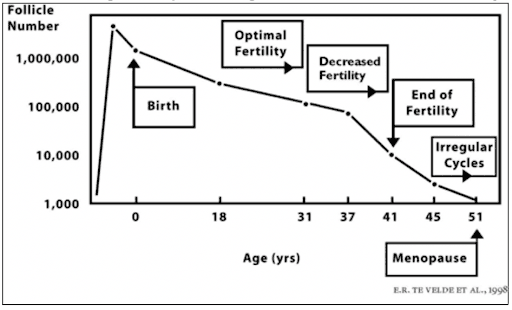
Women are born with all the eggs they are ever going to have, and they don’t make any new eggs during their lifetime. Women are born with approximately two million eggs in their ovaries, but about eleven thousand of them die every month prior to puberty. As a teenager, a woman has only three hundred thousand to four hundred thousand remaining eggs, and from that point on, approximately one thousand eggs are destined to die each month. This phenomenon is completely independent of any hormone production, birth control pills, pregnancies, nutritional supplements, or even health or lifestyle. Nothing stops this inexorable death of approximately one thousand eggs every month regardless of ovulation, ovarian inhibition, or stimulation. Whenever the woman runs out of her supply of eggs, the ovaries cease to make estrogen, and she goes through menopause. Despite a lot of journalistic hype, there is no similar phenomenon in men. Men continue to make sperm and testosterone at virtually the same rates, with only a very modest diminution as they age.
Many population studies have demonstrated over several decades that the average fertile woman becomes infertile by age 35-40, and undergoes menopause by age fifty. The mean age of the end of female fertility (according to all the early population studies of fertile women) precedes menopause by about ten to thirteen years. The end of fertility for an otherwise normal, fertile woman, and the age of the onset of menopause, correlates strictly with the decline in the number and quality of eggs remaining in her ovary.
The average female life expectancy in the Western world is currently about eighty-four, whereas in 1900, the average life expectancy was fifty, and in 1850, it was only forty-two years of age. Meanwhile, the average age at which young girls start menstruating in the modern world has decreased from age thirteen or fourteen to age ten or eleven. Neither the overall life expectancy nor the age of menarche (the beginning of menstruation) has any effect on the average age of menopause. In fact, the average age of menopause in almost every population studied over any period of time and in any era has remained constant at around fifty. Although some women go through menopause in their twenties (because of POF, i.e., premature ovarian failure) and some go into menopause in their late fifties, the timing does not appear to depend upon any specific element in their lives other than the number of eggs with which they were endowed at birth.
It is this wide variation in the endowment of eggs from woman to woman that will determine whether you will lose your fertility early (late twenties or early thirties), or whether you’ll be one of the lucky women who is able to have children into her mid- or even late forties. To recap, the average woman will have three hundred thousand to four hundred thousand eggs at the time of puberty. An average of one thousand will die every month, and only one of those thousand every month is destined to ovulate. By age 35-37, the average woman will be down to only about twenty-five thousand remaining eggs. When only twenty-five thousand eggs remain in the ovaries, menopause will occur in approximately thirteen years. Thus, the average woman begins to become infertile by age thirty-seven or earlier, when her ovarian reserve goes down to about twenty-five thousand eggs, and at age fifty, she will go through menopause. But there are wide variations from this average. What you need to know, in order to plan your entire life.

It is often erroneously thought that just one follicle develops every month, during the first two weeks of the cycle, ultimately culminating in a large, twenty-millimeter follicle from which the egg is ovulated at approximately day fourteen (in a typical twenty-eight-day ovulatory menstrual cycle). Development of this single, dominant follicle every month with its increasing production of estrogen, and the entire regulation of the monthly cycle via the pituitary hormones of FSH and LH, only gives a tiny part of the picture; it only shows what is happening to one egg in an ovary that contains, in a fertile young woman, as many as 200,000 eggs. That one egg that was destined to ovulate, developed as the single dominant follicle out of the thirty or so much smaller pre-antral and antral follicles, which had been developing in the ovary for as long as four months prior to the beginning of the current twenty-eight day menstrual cycle.
Most of the ovaries’ 300,000 to 400,000 follicles are quiescent and doing nothing during any given month, but out of that primordial pool, a certain number (an average of thirty to forty) will begin to develop each day. During the first four months of a follicle’s development, it is completely independent of any hormonal influence. FSH and the monthly hormonal cycle have no influence yet. Sometime between 0.2 millimeters and 2 millimeters in size, these so-called antral follicles begin to become sensitive to stimulation by FSH from the pituitary gland. Prior to the time when these tiny follicles finally become ready to enter the current menstrual/ovulatory cycle, they are completely unaffected by whatever hormonal events have been taking place in the previous cycles.
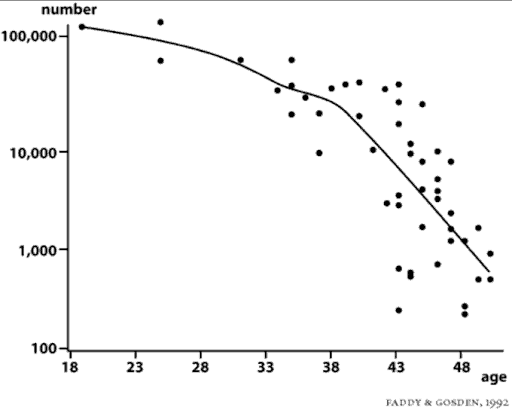
As previously stated, the number of follicles leaving the resting pool (destined to become either the lucky egg that is ovulated, or the unlucky ones that undergo atresia, i.e., cell death) may average about thirty per day, or one thousand per month, and that number is related to the age of the woman, and to her declining fertility. Thus, when a woman is only twenty years of age, an average of thirty-seven follicles per day leave the resting stage. When she is thirty-five years of age, an average of ten follicles per day leave the resting stage, and when she is forty-five years of age, an average of two follicles per day leave the resting stage. This means that the number of follicles per day that begin to become antral, and thereby capable of rescue from death by FSH stimulation, is inversely related to the age of the woman. The younger the woman and the larger the total number of eggs in her ovaries, the greater the number of eggs in any given month, or any given day, that will leave the resting phase and develop into antral follicles (of which only one per month is destined to ovulate; all the others will die).
Ovarian Tissue, Embryo, and Sperm Freezing
Our cryopreservation (sperm, egg, embryo, and ovarian tissue storage) program is one of the best in the world. We have a 65% pregnancy rate per cycle with frozen embryos, which is no different than for fresh, or unfrozen, embryos. Our frozen embryo survival is over 99%. Therefore, if you have “extra” viable embryos resulting from your IVF procedure, you can feel comfortable allowing us to freeze them. Please see our section on mini-IVF on egg freezing, and on cancer for more information as already mentioned, we have brought successful egg freezing to the United States. But our entire program for freezing is more diverse and experienced than any other IVF program in the world.
We offer egg freezing for women who wish to delay childbearing, and who want to preserve their fertility for the future. Although the freezing of embryos has been quite reliable, the freezing of eggs was for a long time only experimental, and, until recently, not very successful. However, the Infertility Center of St. Louis has begun a partnership with Tokyo to bring an exciting new technique, egg vitrification [see technical video], to the United States. With this method, we can ‘flash-freeze’ unfertilized eggs with remarkable effectiveness, and then thaw them at any later date for re-implantation. We can also offer ovarian tissue freezing for women who do not want to undergo IVF in the future but who just want to conceive naturally or, of course, for cancer patients.
The classic problem with freezing eggs used to be that as one lowered the temperature below the freezing point, the egg’s uncombined genetic material (ready for fertilization, but also in a complex and delicate state) would suffer damage due to ice crystals forming inside the cell. It was only possible to freeze embryos, in which the genetic material had already combined with that from the sperm, and stabilized. The classic freezing techniques (which have been known since 1983) were based on trying to extract water from the cell as the temperature drops, to minimize ice crystal damage. This has all changed now with the development of our new vitrification techniques. We no longer have to play a tenuous game of minimizing ice crystal formation — we can now entirely avoid it — so that there is no internal damage to the egg whatsoever.
We also offer very excellent quality ovarian tissue freezing. This procedure can be used to preserve the possibility of future fertility for women who are about to undergo radiation or chemotherapy for cancer. It may also be used for those women who may not be able to plan for children until they are older. We have had many successful ovarian tissue transplants and pregnancies, and so ovarian tissue freezing has excellent promise for preserving your natural fertility.
Sperm freezing may be needed if the husband is about to undergo radiation or chemotherapy for cancer, or is being deployed to a war zone, but wants to father children later. In addition, sperm may be frozen if the husband has no sperm in his ejaculate and requires a onetime surgical sperm extraction procedure, or simply, if the husband is afraid that on the day of your IVF procedure he may be too nervous to provide a specimen. Again, you can be very confident in the reliability of our sperm freezing and storage system.
Freezing Eggs or Embryos by the Vitrification Process
This new technique of freezing called “vitrification” avoids the damage caused by ice forming inside the cell by not trying to pull every last molecule of water out because it is impossible to do this 100%. In fact, 70% of the cell is water, and at best you can reduce that to 30%. So with the conventional controlled rate slow-freezing technique, there is always going to be some intra-cellular ice crystal formation, causing some damage to embryos, and severely damaging most eggs. Vitrification uses a super high concentration of antifreeze (DMSO and ethylene glycol) and drops the temperature so rapidly that the water inside the cell never becomes ice. It just instantaneously super-cools into a solid with no ice crystal formation at all.
We can now freeze and thaw, and even refreeze and re-thaw, with impunity, using this new protocol from Dr. Masashige Kuwayama from Tokyo. With conventional “slow freezing,” the temperature of the embryo goes down at precisely 0.3°C per minute. With vitrification (using four times the concentration of antifreeze, or cryoprotectant), the temperature is dropped at 23,000 degrees C° per minute, that is 70,000 times faster. At that speed of cooling, and at that concentration of antifreeze, ice crystals simply cannot form.
Of course, it is not quite as simple as it might sound. Such high concentrations of antifreeze, in a few minutes, could be toxic to cells due to osmotic shifts. Therefore, the embryos (or eggs) must first be placed in lower concentrations of antifreeze (and sucrose to draw some water out) and then left in high concentrations only for less than a minute before instantaneous freezing. Then when the time comes to thaw the embryo, it must be instantaneously warmed, immediately taken out of the high concentration of antifreeze, and then placed into a solution with a lower concentration, in order to avoid antifreeze osmotic toxicity. This requires more skill than conventional freezing, but it is faster, cheaper, and most importantly, avoids almost all freezing damage to either eggs or embryos. Such a reliable method of embryo freezing gives the IVF program much greater ability to avoid dangerous multiple pregnancies, and makes scheduling for procedures like egg donation simpler for the patient.
Using this vitrification technique for freezing, we can reliably preserve eggs as well as embryos so that the pregnancy rate is no different than if the eggs or embryos had never been frozen. This allows us to preserve the fertility of young women for the future if they wish to delay childbearing, but not lose their fertility as they age.
There are many procedures that can be used to preserve the fertility for women who are about to undergo radiation or chemotherapy for cancer. Dr. Silber has wrote this article on Fertility Preservation for Cancer Patients published by the Journal of Cancer Biology & Research.
If you have any questions, you may call us at (314) 576-1400.