Dr. Silber Explains Fertilization Rates
Each Patient Has Their Own Incubator
Dr. Silber Explains
IVF (in vitro fertilization) is the most successful form of ART (Assisted Reproductive Technology). If the fallopian tubes are damaged or the sperm is poor, it is obviously the only acceptable treatment. It is also usually the most effective treatment for most other types of infertility as well. The eggs are fertilized in our laboratory, and the resulting embryos are then placed into the uterus 3 to 5 days later. This procedure achieves remarkable pregnancies even in women with hopelessly damaged fallopian tubes, seemingly sterile husbands, PCOS, and even “unexplained” infertility. Problems with the husband’s sperm are never a serious issue since we can fertilize the eggs with ICSI (intracytoplasmic sperm injection). In fact, in our program, we routinely use ICSI in all cases to guarantee against any risk of failed fertilization. Our IVF pregnancy rate is over 60% per attempt, and including frozen embryos from one attempt approaches 94%. This is regardless of diagnosis, and we accept all of the most difficult cases. Extra embryos are frozen and saved for a later, much less expensive future pregnancy. Our very special Japanese freezing technique essentially assures no damage to viable embryos. Therefore, the success rate for frozen embryo transfers in our program is just as high as for “fresh” IVF transfers, or in many cases, frozen embryo transfers are actually much higher than fresh. See: ART Pregnancy Rates
Learn more about IVF for older women
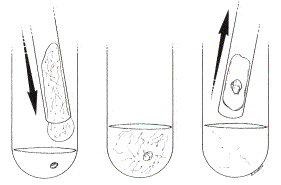
With IVF, the eggs are fertilized in the laboratory under perfect conditions that exactly simulate your Fallopian tubes and uterus.
It is best to look for IVF in a setting of a major quality medical center that is more concerned about the practice of good medicine than about your pocketbook and which has invested heavily in high standards “clean room” air purification systems, the best equipment, most skilled doctors, embryologists, nurses, ultrasonographers, and kind & communicative IVF coordinators.
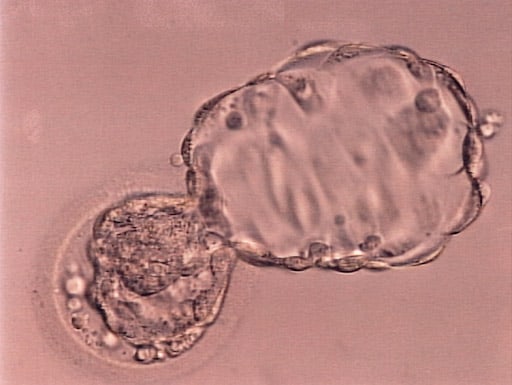
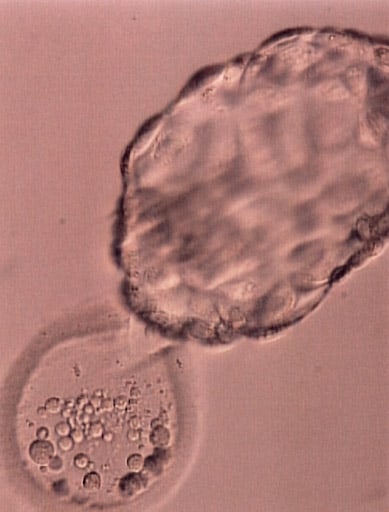
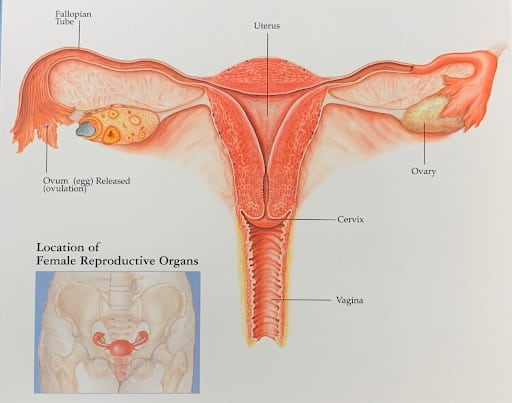
These illustrations show how a fertile woman would normally get pregnant.
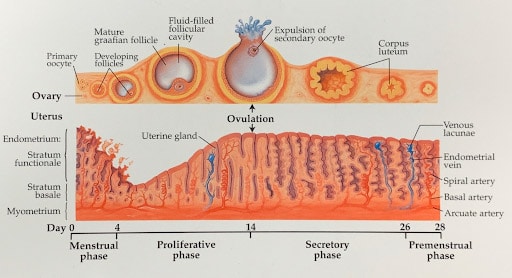
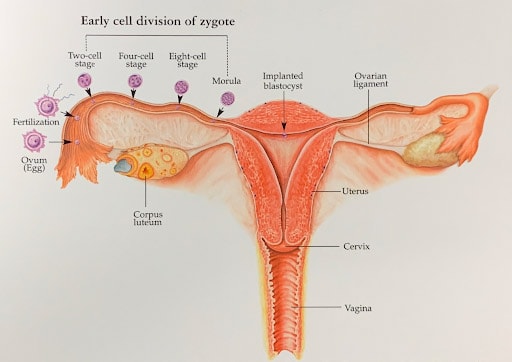
After ovulation, the egg is released from the ovary, and grasped by the fimbriated end of the Fallopian tube. When the egg is ovulated at mid-cycle, the uterine lining undergoes a precisely timed “secretory” change directed by the ovary beginning to secrete progesterone. The egg is then fertilized by sperm that travel through the uterus into the Fallopian tube. As the embryo develops to blastocyst, it then enters the uterus and implants. IVF bypasses all this.
How Does In Vitro Fertilization (IVF) Work?
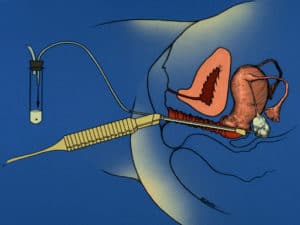
Eggs are retrieved by ultrasound-guided needle aspiration under light sedation (in the operating room). This involves no surgical incision, and virtually no pain afterward. You just leave the hospital directly from the operating room, with no pain, and come back three to five days later to have the embryo (or embryos) placed very simply into the uterus through the cervix with a tiny catheter. No incision and no anesthetic are needed. An hour later you can go home. There is no pain from the procedure. Often all the embryos will be frozen (with no harm to them) so that they can be transferred to you when your uterus is most receptive. In order to retrieve these multiple eggs for IVF, the woman must undergo injections with hormones and careful monitoring of her ovaries by ultrasound and her hormone levels by blood tests every day or every other day until she is ready for the egg retrieval. However, our “Mini-IVF” technique is very easy and pleasant as compared to regular conventional IVF. This can at first seem very intimidating, but we will “hold your hand” throughout the whole time and guide you gently through this. There are several methods of hormonal stimulation for IVF, that basically can be divided into what we call “conventional stimulation” and “minimal stimulation,” or “Mini-IVF.” We will explain which approach is most suited for your particular situation, althoughMini-IVF is usually the best method for most patients. It is easier on the patient, less expensive, and actually higher pregnancy rates. Meanwhile, you can find out more about this by reading our pamphlet, by watching our ART teaching DVD, or in more depth by reading my book, How to Get Pregnant [links to Amazon.com].
Related Media
Mini-IVF: Minimal Stimulation IVF Technique
Mini-IVF works well not only in reducing costs and having superior results in older patients, but also gets us a 4-5 times higher baby rate per egg in younger patients with better prognosis as well. Mini IVF is simple for the patient, but complex for the physician, therefor only a few physicians do it.
In addition to IVF with conventional stimulation, we enthusiastically offer and prefer IVF with minimal stimulation (Mini-IVF), which is a new, dramatically lower-cost option with better results. OurMini-IVF protocol, first conceived in Japan, is truly an amazing breakthrough. When patients contemplate IVF, their first reaction is often the fear of daily injections of hormones for months, the incredibly high cost of the drugs, the risk of multiple pregnancy and consequent prematurity, side effects related to high levels of estrogen resulting from large numbers of eggs, hyperstimulation syndrome, and the prospect of painful daily injections. Mini-IVF is a very unique approach developed by our colleagues in Japan (and then perfected in our clinic) to circumvent these problems and to simplify IVF for patients, reducing the cost while maintaining the same or better success rates. For older patients, the success rate is much higher withMini-IVF than with conventional IVF. With the refinements we have added toMini-IVF, the pregnancy rates are startlingly high.
Infertile couples from all over the world come to St. Louis, Missouri, to chase their dream, because Dr. Sherman Silber and his team are simply the best there is.
Discovery Health Channel Documentary

Mini-IVF is designed to recruit not as many (but high quality) eggs, thus avoiding the risks of hyperstimulation, reducing the number of injections and dramatically reducing the cost of medications. In many patients who had very poor quality embryos with conventional IVF stimulation protocols,Mini-IVF dramatically improved their embryo quality and resulted in pregnancy in otherwise “hopeless cases.” This approach is not just a simple-minded reduction in hormonal stimulation. It is an ingeniously conceived and completely different approach to IVF that improves egg quality and saves the patient much of the complexity and cost associated with more conventional IVF protocols. Here is how it works: On Day 3 of the menstrual cycle or Day 6 of birth control pills, you start on a low dose of Clomid (50mg), but you don’t stop the Clomid in five days as is usually the custom. You just keep taking the Clomid until ultrasound monitoring shows the follicles to be ready for ovulation. A very tiny “booster” dose of gonadotropin (just 150 iu of FSH), is added every other day. Clomid not only stimulates your pituitary to release FSH and LH naturally (by blocking estrogen’s suppressing effect), but also staying on the Clomid (a unique new approach) blocks estrogen’s stimulation of LH release, and so also prevents premature ovulation. Thus, with this simple change in protocol, the old-fashioned, cheap Clomid can stimulate the development of a smaller number of better quality eggs for IVF and also prevent premature ovulation. Another advantage of this protocol is that you did not have to go on Lupron first to suppress the pituitary.
Successful IVF is easy for the patient, but complex for the physician, that’s why most don’t do it.
Staying on Clomid blocks estrogen from stimulating your pituitary to release LH, and this prevents premature ovulation without your having to be suppressed. This means that you can be induced to ovulate with just a simple injection or nasal sniff of Lupron. This causes a more natural LH surge and avoids the prolonged negative effect of HCG. The next step is to recognize that Clomid temporarily inhibits the uterine lining (because it prevents estrogen from stimulating the endometrium). That is one reason why results in the past have been so poor with the use of Clomid for ovarian stimulation. The embryos are less likely to implant in such endometrium. But that problem is solved by using the Japanese protocol for embryo freezing, “vitrification.” Using this approach, we can now very safely freeze embryos with virtually no risk of loss. Frozen embryo transfers can then be performed in later natural cycles. Even for poor prognosis cases of older women with low ovarian reserve, there is an advantage toMini-IVF over high dose stimulation. Such patients normally yield very few eggs even with huge megadoses of gonadotropin. Mini-IVF is just as likely to yield as many eggs as giving huge megadoses of gonadotropin. But the egg quality is better and they can afford to do more cycles if that is what is required in older women or poor prognosis cases. The baby rate per egg is 4 to 5 times higher. Think of this simple parable: If you are sitting under an apple tree, and wish to eat the ripest and ready apples, you have a choice. You can chop down the tree, and look at every apple on the fallen tree to see which ones were ready. Or you can simply try to shake the lower branches and eat the one or two that have fallen. That is the idea of mini-IVF. For many patients, it will remove much of the aggravation and complexity associated with IVF, and also dramatically reduce the cost and increase the success rate. But it requires a great deal of sophisticated ability to do it well. It’s remarkable how many poor quality eggs are obtained in most conventional IVF cycles and how few of them will ever become a baby. WithMini-IVF, we get a somewhat smaller number of eggs, but most of them make perfect embryos. So the pregnancy rate per egg has been shown to be fivefold higher withMini-IVF than with conventional stimulation, and the pregnancy rates are just remarkable. We recently had a 31 year old patient with premature menopause. She had hot flashes, and night sweats from low estrogen, and a menopausal FSH level of 109 with an AMH of .008. Yet withMini-IVF we obtained two eggs and two beautiful embryos. Because she is only 31 years old, that is all she needs to get pregnant and have babies. Mini-IVF is tricky to perform well and many centers which try it are deficient. There is no margin for error.
Reasons why Mini-IVF is superior to conventional IVF:
There are several reasons for the success we have with these much lower costMini-IVF techniques which we have pioneered in the U.S. Firstly, it is hard to overstate how crucial the purity of air quality in the lab, as well as the operating room, is. There are organic volatile toxins in the air everywhere in microscopic quantities that don’t seem to affect our well being. But they do dramatically affect the well being of these highly exposed embryos. Secondly, the very clever Japanese approach to minimal stimulation allows us to retrieve smaller numbers of better quality eggs than the more expensive massive dosing of conventional IVF protocols, better quality eggs at a lower cost. Finally, the ability to freeze the embryos with impunity and then transfer in a later cycle where the uterine lining is more perfectly synchronized to the stage of embryo development than during a stimulated cycle, all add up to high success rates at a lower cost.
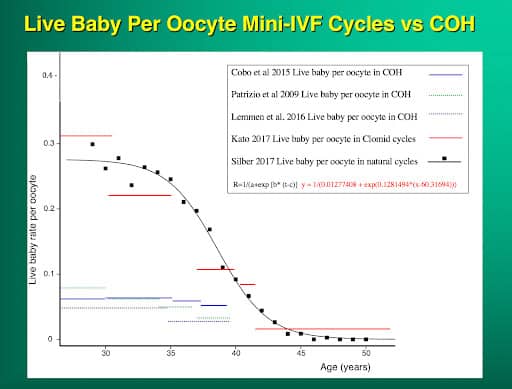
Take a look at this graph showing live baby rate per egg with Mini-IVF compared to conventional ovarian stimulation. The baby rate per egg is 4 to 5 times greater with Mini-IVF at any given age. Even at older ages, the baby rate per egg is four to five times higher than with high dose hyperstimulation. Please note, we use now use Mini-IVF for all patients, good prognosis and poor because at any age the baby rate per egg is 4-5 times higher.
Improvements in Embryo Culture
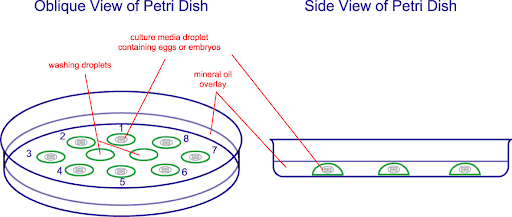
A major improvement in embryo culture was realizing that the oxygen content in the air we breathe is much too high for eggs and embryos. Most cells in the body are exposed to a much lower concentration than the air we breathe. Too much oxygen delivered to these cells can, in a sense, overheat the cell. So it is much better to culture the embryos, not only in 5% CO2 but also in only 5% oxygen (not the 20% that is in the air). This is difficult to do. Large amounts of pure nitrogen gas have to be blown constantly through the incubator at a carefully controlled rate to lower the oxygen concentration in the incubator. But it is worth that extra effort to get higher pregnancy rates. Classically, most IVF labs have cultured embryos at a pH of 7.4 (the normal acid-base of blood concentration), and at an oxygen concentration of 20% (the same as in the air we breathe). However, these are not the acid or oxygen concentrations that are most favorable for embryo growth and development. The acid concentration inside the embryo is normally much greater than that, and the oxygen concentration is much lower. Conventional IVF culturing conditions, therefore, are too alkaline and too oxygen-rich. The oxygen concentration in the Fallopian tube is only about 8% (not 20% as in air), and in the uterus, it is as low as 2%. This type of optimal culturing of embryos requires a lot of extra attention. To reduce the oxygen concentration in the incubator from 20% to 5% requires blowing through a huge amount of nitrogen (95%), and to keep the pH acid at 7.2 (but not too acid below 7.2), requires careful monitoring of the acidity of the media. This represents a lot of extra work, but it is well worth the effort. The better your pregnancy rate per cycle, the less is your eventual cost.
In a high-quality referral hospital setting such as ours, the most rigid air quality system is in place, preventing particles of volatile organic compounds from entering the environment where your embryos are growing in culture. The air around all of us is filled with these toxic compounds in low concentrations that don’t seem to affect your body’s overall health in any obvious way but do seriously affect the growth and development of your eggs and your embryos in culture. We can see the obvious negative effect of non-perfect air quality in an IVF lab on the evolution of poor quality embryos that give lower pregnancy rates than the good quality egg and embryo growth from those same women whose embryos are cultured in high air quality environments.
Only large IVF centers in high-quality hospitals that invest many millions of dollars into “clean room” air quality, can ensure the proper environment for the growth in vitro of your eggs and embryos. Even older women in their late 30’s and 40’s, whose embryos cannot tolerate the slightest stress, develop good quality embryos in a laboratory environment like ours that is free of these common toxins in the air that pervade most office-based settings.
Freezing Embryos by Vitrification
This new technique of freezing called “vitrification” avoids the damage caused by ice forming inside the cell by not trying to pull every last molecule of water out because it is impossible to do this 100%. In fact, 70% of the cell is water, and at best you can reduce that to 30%. So with the conventional controlled rate slow-freezing technique, there is always going to be some intra-cellular ice crystal formation, causing some damage to embryos, and severely damaging most eggs. Vitrification uses a super high concentration of antifreeze (DMSO and ethylene glycol) and drops the temperature so rapidly that the water inside the cell never becomes ice. It just instantaneously super-cools into a solid with no ice crystal formation at all.
We can now freeze and thaw, and even refreeze and re-thaw, with impunity, using this new protocol from Dr. Masashige Kuwayama in Tokyo. With conventional “slow freezing,” the temperature of the embryo goes down at precisely 0.3°C per minute. With vitrification (using four times the concentration of antifreeze, or cryoprotectant), the temperature is dropped at 23,000 degrees C° per minute, that is 70,000 times faster. At that speed of cooling, and at that concentration of antifreeze, ice crystals simply cannot form.
Of course, it is not quite as simple as it might sound. Such high concentrations of antifreeze, in a few minutes, could be toxic to cells. Therefore, the embryos (or eggs) must first be placed in lower concentrations of antifreeze (and sucrose to draw some water out) and then left in high concentrations only for less than a minute before instantaneous freezing. Then when the time comes to thaw the embryo, it must be instantaneously warmed, immediately taken out of the high concentration of antifreeze, and then placed into a solution with a lower concentration, to avoid antifreeze toxicity. This requires more skill than conventional freezing, but it is faster, cheaper, and most importantly, avoids almost all freezing damage to either eggs or embryos. Such a reliable method of embryo freezing gives the IVF program much greater ability to avoid dangerous multiple pregnancy, allows ingenious new protocols like mini-IVF to work with less expense to the patient, allows the patient to have many more chances for pregnancy in subsequent cheaper frozen embryo cycles, and makes scheduling for procedures like egg donation or gestational surrogacy much simpler for the patient.
Using this vitrification technique for freezing, we can now also preserve eggs as well as embryos and sperm. This allows us to preserve the fertility of young women or cancer patients for the future in egg banks if they need to delay childbearing.
Blastocyst Culture
Whenever IVF or ICSI is performed, embryos may be cultured for two, three, even five or six days, before transferring them back into the woman. In order to culture the embryos for five or six days, i.e. to what we call the “blastocyst” stage, you need to use “sequential” culture media systems. We have used such a system since the summer of 1997 because it gives us the option of culturing the embryos for as long as is clinically appropriate for each particular patient’s situation. However, there is a great deal of debate, and some considerable commercial hype, centering around whether to culture for two days, three days, or five or six days. The media we use allows us to culture the embryos to whatever number of days is appropriate for the particular patient. For some patients with poor quality embryo development (a condition which is programmed into the genome of many infertile women), even with the best culture media, the embryos may be better off going directly into the women at day 3. For the average patient, day three transfer to the uterus may be best. For some patients, day 5 or 6 transfer to the uterus may be a good option. The problem with extended culture to day 5 is that there may be a loss of some embryos that might have “made it” if they had been transferred earlier. The major advantage (despite the already mentioned disadvantage) of day 5 or 6 transfer is embryo selection. The implantation rate per day 5 or 6 blastocyst transfer is greater than for transfer of day 2 or 3 embryos. But only 20 percent to 50 percent of day 3 embryos can develop in vitro to day five or six no matter how perfect the in vitro culture system. There is a potential loss therefore of what could have been viable embryos. So selection is the only advantage of blastocyst culture, and this selection has nothing to do with the “quality” of the baby, but rather just whether the embryo “makes it” or not to become a baby. The baby rate per egg is higher with day 3 transfer, the baby rate per embryo is higher with blastocyst transfer. Either way, because our system of embryo freezing is so good, we do not lose anything if we transfer fewer embryos to reduce your risk of triplets or quadruplets, and just save the extra embryos for a later pregnancy. We can even transfer just one embryo at a time (if that is your wish) without at all reducing your chance of pregnancy.
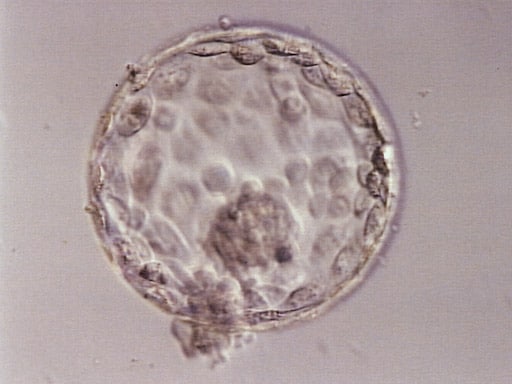
Assisted Hatching
Another attempt at improving the pregnancy rate is the idea of “assisted hatching”. The concept of this procedure is that based on the fact that the embryo normally sits around in the uterus without any effort to implant until around day six. Until day six, the embryo keeps growing within its very tough zona pellucida (outer shell). But on day six, that zona pellucida begins to thin out, and the embryo then eventually “hatches,” just like a chicken out of an egg. It is at this moment of hatching that the embryo, now called a “blastocyst,” actually implants into the uterine lining, the endometrium. It is at this moment, around day eight after fertilization, that pregnancy actually occurs, and this free-floating ball of cells finally becomes a part of the mother. One theory to explain the perplexing phenomenon that many IVF embryos do not result in pregnancy has been that this thinning of the zona and hatching of the embryo may be defective and in some way impeded in embryos that have been cultured in vitro. The solution to this problem would be to microsurgically thin out the wall of the zona pellucida of these embryos on day three. It was hoped that this micro-manipulative process to the embryos might provide a necessary extra bit of help to improve the implantation rate. This proposition is very difficult to prove with certainty, but in many centers, including ours, assisted hatching is performed on most embryos, and the results have thereby improved. It is a beautiful procedure and a wonderful rationale, and in women who have failed to get pregnant through the transfer of perfectly good embryos, the standard approach at the present should be to give them the benefit of the doubt and to do assisted hatching. However, it is routine and there should not be any extra charge for doing it.