SHERMAN J. SILBER* AND H. EDWARD GROTJAN†
From the *Infertility Center of St Louis, St Luke’s Hospital, Chesterfield, Missouri; and †Serono, Inc, Rockland, Massachusetts.
American Society of Andrology, November/December 2004
ABSTRACT
Between 1975 and 2003, a total of 4010 patients have undergone microscopic vasectomy reversal by 1 surgeon at 1 institution. Of these cases, 3904 had available records and 1735 were successfully contacted to obtain reliable long-term data. A total of 1556 (89.7%) were able to establish a pregnancy in their female partner, resulting in 2111 pregnancies. When there was no sperm in the vas fluid at the time of vasectomy reversal, vasoepididymostomy was performed rather than vasovasostomy. A total of 1581 patients underwent bilateral vasovasostomy; 1184 underwent vasoepididymostomy on one side and vasovasostomy on the other side; and 1139 underwent bilateral vasoepididymostomy. Of patients undergoing vasovasostomy, 2.1% had no sperm in the ejaculate postoperatively, and 10.3% of patients undergoing bilateral vasoepididymostomy had no sperm in the ejaculate postoperatively. Thus on average a patency rate of 96.2% in the total group of patients was achieved. When the vasectomy was less than 10 years prior to reversal, the patency rate was 98%. When the vasectomy was 10 or more years prior to reversal, the patency rate was 93%. Among all the patients, 77.7% had sperm counts greater than 5 million/mL postoperatively. Among patients undergoing bilateral vasovasostomy and those undergoing bilateral vasoepididymostomy, 92.5% and 84.3% eventually achieved a pregnancy, respectively. The pregnancy rate did not differ with patients that had a sperm count of greater than 5 million sperm/mL. The pregnancy rate for less than 5 million sperm/mL was 78.3% and when there was greater than 5 million sperm/mL the pregnancy rate was 91.9%. Although the duration of time between vasectomy and vasectomy reversal had an impact on pregnancy rate, the greatest impact was the age of the wife. Among wives under age 30 at the time of the vasectomy reversal, 94.2% established a pregnancy, but only 61.1% of wives age 40 or older established a pregnancy. We conclude that microsurgical vasectomy reversal is preferable to sperm retrieval and intracytoplasmic sperm injection (ICSI), since the pregnancy rate appears to be higher with this technique than with sperm retrieval and ICSI. It does not appear that sperm antibodies or testicular damage are likely to account for failure to achieve pregnancy after vasectomy reversal. Rather, it is likely to be related to partial or complete obstruction following surgery, or to the fertility of the female partner.
Prior to the mid 1970s, when microsurgical vasovasostomy was first introduced, vasectomy had been generally regarded as not reversible and attempts at reversal yielded very poor results (O?Connor, 1948; Derrick et al, 1973; Derrick and Frencilli, 1974). In 1948, some 135 surgeons who were polled had tried to perform vasovasostomy and reported a very poor prognosis for return of any sperm to the semen (O?Connor, 1948). In 1973, of 1630 cases performed by 542 urologic surgeons, the patency rate for vasovasostomy was only 20%, and pregnancies were considered to be unusual (Derrick et al, 1973). Attempts to improve those poor results in the 1960s and early 1970s with pull-out sutures, gold valves, magnetic ball valves, and removable silicone plugs failed to improve the dismal results (Derrick and Frencilli, 1974). Later attempts in the 1990s to use stents to improve success rates of vasovasostomy without having to resort to accurate microsurgical anastomosis have also failed (Rothman et al, 1997). The literature on this issue can be very confusing because of the relatively small number of patients (often less than 30) in most published series (Middleton et al, 1987; Fox, 1994, 1997; Matsuda et al, 1994; Witt et al, 1994; Chiang 1996; McDonald, 1996; Yamamoto et al, 1997; Carbone et al, 1998; Inaba et al, 1999; Jokelaine et al, 2001; Schrepferman et al, 2001; Huang et al, 2002).
There are a remarkable number of heated controversies on this subject. Is microsurgery preferable to macrosurgery? What is the cause for nonpatency, blockage at the vasovasostomy site, or in the epididymis? What is the cause of failure of the wife to get pregnant despite patency? Do antibodies play a role? Is there testicular damage from pressure buildup? What is the effect of the wife’s fertility? Why is there a discrepancy between patency after surgery and the wife’s achieving pregnancy? What is the effect, if any, of partial vas blockage, partial epididymal blockage, or epididymal malfunction? What effect does the time since vasectomy or absence of sperm in the vas fluid have? Should we do only vasovasostomy at the first operation, or if there is no sperm in the vas fluid, should we perform vasoepididymostomy? Should an accompanying varicocele be repaired?
In fact, should we actually do vasectomy reversal at all, or just subject all these patients to sperm retrieval and intracytoplasmic sperm injection (ICSI)? Should epididymal sperm be frozen at the time of vasectomy reversal? Is a sperm granuloma at the vasectomy site harmful or helpful? How has the changing technique for vasectomy over the last 30 years impacted the success rate for vasovasostomy or the need for vasoepididymostomy? Should vasectomy reversal be performed if the patient’s wife is older, or should they just go right to ICSI with sperm retrieval? These controversies have sometimes been remarkably hostile (even in the written literature) and emotional. We will try to address these controversies by reporting our results in an enormous series of over 4000 cases performed by one of us (S.J.S.) over the last 30 years comparing our early reports in the 1970s and 1980s with our current accumulated results in 2004 (Silber, 1975, 1976, 1977a,b, 1978a,b, 1979, 1980a; Friend et al, 1976; Owen, 1977).
Our procedure over the last 3 decades has been based on results reported in our earlier papers in the 1970s. At that time, we originally performed vasovasostomy without vasoepididymostomy for all patients regardless of the quality or appearance of sperm in the vas fluid at the time of the reversal. However, our data in 1977 demonstrated that when there was no sperm in the vas fluid (with the exception of the fluid being crystal clear), patients remained azoospermic even after a very accurate vasovasostomy. Therefore, in the cases reported here, we routinely performed vasoepididymostomy rather than vasovasostomy on either side or both sides when no sperm was found in the vas fluid. Furthermore, since our data in 1977 demonstrated no difference in results between patients who had a previous failure at vasectomy reversal versus patients undergoing their first vasectomy reversal at our center, we grouped all the data together regardless of whether the patient had a previous failed attempt at reversal. In addition, if there was a varicocele discovered on physical examination prior to vasectomy reversal, varicocelectomy was not performed.
Patient Population
A total of 4010 patients were referred to one of us (S.J.S.) over the last 30 years to undergo microsurgical reversal of vasectomy. Records were available for this study on the last 3904 of these patients. The average age of these men was 40.0 ± 7.1 (SD) years, and the average age of wives (known in 3793 of the cases), was 31.0 ± 5.0 years. 1581 (40.5%) of the patients underwent bilateral vasovasostomy, 1184 (30.3%) underwent vasoepididymostomy on one side and vasovasostomy on the other (unilateral vasoepididymostomy), and 1139 (29.2%) underwent bilateral vasoepididymostomy. On either side, patients with no sperm in the vas fluid (if the fluid was not crystal clear), underwent vasoepidymostomy instead of vasovasostomy. A total of 802 of the patients (22.3%) had a previous failed vasectomy reversal and were coming here for a rereversal.
The average time between vasectomy and vasectomy reversal was 10.0 years 6 5.4 (SD). Observations recorded at the time of the surgery included the age of the patient, the age of wife, the duration of time since vasectomy, the gross appearance of the vas fluid, the microscopic analysis of the vas fluid for sperm or sperm parts, and the type of anastomosis, whether vasovasostomy or vasoepididymostomy. The number of years since vasectomy was known and was recorded in 3591 cases. Postoperative semen analysis was available in 3378 (86.5%) of the cases.
Over a 6-month period of time every single chart was reviewed, and 2 counselors worked full time attempting to contact these 4010 patients. This procedure was somewhat facilitated by modern internet search capabilities. Many patients during these 30 years had moved, and phone numbers were no longer valid, requiring a thorough internet search. The purpose of this telephone campaign was to fill in any missing detail about pregnancy or lack of pregnancy, at any period of time subsequent to the vasectomy reversal. Not only was pregnancy data thus updated but the delivery of a baby (the ultimate end-point in modern fertility outcome studies) was ascertained. In that respect our data follow-up approach was modeled after that described by Fuchs and Burt in 2002 (Fuchs and Burt, 2002). In their report, 48 cases out of 173 were dismissed because of lack of inability to contact the patients, and 9 were dismissed because apparently the patients were not attempting to achieve pregnancy. Also there was no clear distinction between unilateral vasoepididymostomy and bilateral vasoepididymostomy. It became obvious that follow-up information is difficult to obtain in this population of patients, and it requires an intensive telephone campaign to validate and update data.
Of the 3904 patients in which records were available, including postoperative sperm count in 3591 patients, in only 1735 patients was it possible to obtain long-term follow-up on the eventual occurrence or nonoccurrence of pregnancy or delivery of a baby. Thus data were accumulated on surgical technique, demography of patients, and subsequent sperm counts in 3904 patients, and reliable pregnancy information was available in 1735 cases. Comparisons were made to results related to the age of patient, the age of the wife, the time since vasectomy, the resulting sperm count, and the type of anastomosis whether vasovasostomy or vasoepididymostomy. This represents the first detailed and massive follow-up study of vasectomy reversal performed by 1 operating surgeon.
Microsurgical Technique

Our microsurgical technique over the last 30 years for vasovasostomy is essentially unchanged from our original reports. Our technique for vasoepididymostomy has always been ‘‘specific tubule’’ microanastomosis. We first described this in 1978 using an end-to-end approach (Silber, 1978c). In 1984 we switched from end-to-end to end-to- side specific tubule anastomosis because it seemed to be an easier approach and produced similar results. Therefore, we grouped all of our vasoepididymostomies together regardless of whether an end-to-end or end-to-side procedure had been performed (Silber, 1984). Although the debate may continue about the use of a microscope for vasovasostomy, the issue of whether the surgeon should be adept at microsurgery becomes foolish when it is apparent that vasoepididymostomy may be necessary, and nobody would suggest that vasoepididymostomy can be done properly without a microscope and special microsurgical skill (Donovan, 1995).

From the very beginning of our earliest reports in 1975 there was great resistance to the use of a microscope for vasectomy reversal. We never understood this resistance since the purpose of the microscope, as opposed to loupes or the naked eye, is simply to make it easier to do the operation properly. Use of the microscope should not make the operation more difficult, it should make it easier. Yet many authors persisted in trying to advocate doing this procedure either with the naked eye or with 23 or 33 loupe magnification only. However, if one cannot do a 2-layer microlayer vasovasostomy using an operating microscope, then there is certainly no chance that person can do an adequate anastomosis for vasoepididymostomy (MacDonald and Edson, 1976; Rowland et al, 1977; Lykins and Witherington, 1978; Fenster and McLoughlin, 1981; Fallon et al, 1981; Shessel et al, 1981; Redman, 1982).
After general anesthesia is induced, each scrotal sac is entered through a 1.5 inch longitudinal incision and the scrotal contents are extruded extravaginally and examined. If there is only a small segment of vas missing, a simple vasovasostomy can be performed quite readily under local anesthesia. However, because often a larger segment is missing and because vasoepididymostomy often has to be resorted to, general anesthesia is employed in most of our cases. If a large segment of vas deferens is missing, as is often the case in more recent surgeries because the urologists are very concerned about lawsuits stemming from unwanted pregnancies, the vas must be freed up considerably above and below the area of obstruction to avoid tension on the suture line. Often these incisions have to be extended up to the level of the external inguinal ring and very occasionally even more proximally, in order to free up enough vas deferens proximally so that there will be no tension on the suture line and so that the testicle will sit properly in the scrotal sac.
For vasovasostomy, the proximal and distal end of the vas deferens on either side of the vasectomy site are placed in the jaws of a Silber vasovasostomy clamp (V. Mueller) and drawn into the field of the operating microscope. The 2 ends of the vas are carefully checked at this time to make sure there is no tension. The fibrotic section is then transsected proximally and distally, and all bleeders at the cut end of the vas are cauterized with microbipolar forceps during continuing pulsatile irrigation with heparinized saline. If there is any tension on the 2 ends of vas deferens, we do not try to use a holding suture to pull them together under tension, but rather extend the incision if necessary and free up the vas deferens so that there is no tension. Only if the vas fluid shows no sperm or purely sperm heads do we perform a vasoepididymostomy. If the vas fluid is crystal clear with or without sperm or if the vas fluid has only long-tailed but completely nonmotile sperm we still will perform vasovasostomy. This decision making was based upon the 1977 report of Silber (1977c).
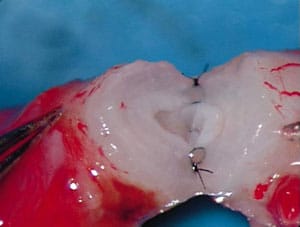
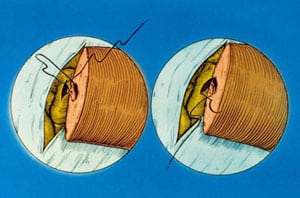
The inner mucosa of the vas is sutured with 10-0 monofilament nylon (Sharpoint DRM 4 needle black monofilament nylon, 70 microns, 1358 curve). The mucosal sutures pick up a small portion of muscularis in order to facilitate the anastomosis (Figure 1). The only reason for doing 2 layers is to be certain that accurate mucosa-to-mucosa approximation is achieved. If this can be done with a 1-layer anastomosis, that is quite acceptable. However, often there is a disparity between the size of the proximal and distal lumens of the vas. The reason for that disparity is that there has been pressure buildup over many years. Throughout the anastomosis visualization is facilitated by continuous pulsatile irrigation with heparinized saline.
We do not delay tying the sutures until after they are all placed. Rather, we tie each interrupted suture as we go in order to avoid what we term a ‘‘marionette puppet show’’ in which sutures can become entangled with each other and confusing, making the operation more difficult. We find that if you tie the sutures as you go and your assistant is skilled at holding the vas open with microforceps, there is no need for double-needled sutures, and the operation is actually easier and more ergonomic. We have not used any ‘‘technical aids,’’ which have been described in the literature such as stents, whether temporary or permanent, or the so-called muscularis inversion technique (Belker, 1982; Donovan, 1995; Fox, 1996). The outer muscularis is then sutured with 9-0 nylon interrupted sutures (Sharpoint, 9-0 nylon black monofilament HSV 6 100 micron vas cutting needle) after 6 inner mucosal sutures of 10-0 nylon have been placed. The end-to- end anastomosis of vas-to-vas is facilitated by using the vasovasostomy clamp, which we simply rotate 1808 when the anterior layer has been finished in order then to suture the posterior side.
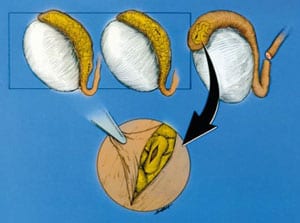
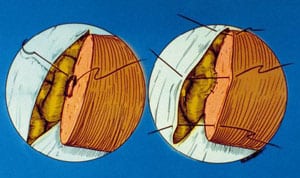
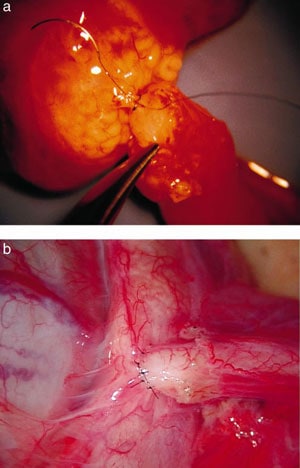
For vasoepididymostomy, the tunica vaginalis is opened and the epididymis inspected under the operating microscope. We begin at the distal cauda epididymis and work proximally until we get beyond the point of secondary obstruction (Figures 2 through 5). This area is ascertained by taking a small window out of the tunica covering the epididymis, dissecting the epididymal tubule in that location, and then making a small longitudinal slit with a microscissors under 403 magnification. The fluid gushing from the tubule is then aspirated with a micropipette, diluted in 1/2 mL of HEPES buffered HTF media, and examined in the operating room under phase contrast microscopy.
Until 1984 we had serially transsected the epididymis until we obviously passed the proximal point of obstruction, but now with the end-to-side approach we make our decision where to perform the anastomosis based upon the presence and quality of sperm in the epididymal fluid. The epididymal sperm, if motile, are then frozen and stored with a standard vapor technique for future use if the operation should prove not to be successful.
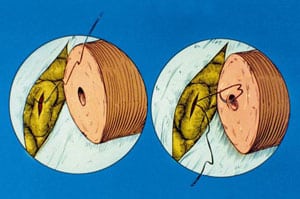
Under low-power magnification the posterior muscularis of the vas is sutured to the posterior epididymal tunic using three 9-0 nylon interrupted sutures. Then the inner mucosa of the vas deferens is sutured to the longitudinal slit in the epididymal tubule, end-to-side using six 10-0 nylon (Ethicon monofilament black nylon 10-0 V75-3 taper cut needle) interrupted sutures. The first interrupted suture is placed at the 6 o’clock position going first from outside to inside of the epididymal tubule, and then inside to outside in the mucosal layer of the vas. In this fashion we then put in the remaining 10-0 nylon interrupted sutures working around anteriorly. Finally, the anterior muscularis of the vas is sutured to the anterior epididymal tunic with 5 more 9-0 nylon interrupted sutures (Figure 6A). None of these cases have been performed on an outpatient basis. In all cases, drains are left in the scrotum, the patient stays in the hospital overnight, and a nurse regularly changes the dressings. The drains are removed the next day and only then is the patient allowed to leave. With this approach there is minimal postoperative pain or risk of hematoma or swelling. Although the popular mode today is to do these procedures on an outpatient basis, patients coming to us who have had previous outpatient vasectomy reversals routinely complain about the amount of discomfort and swelling they endured going home without drains immediately after surgery.
Our current operating time for bilateral vasovasostomy is about 1 hour and 15 minutes, although in the early days it was over 2.5 hours. Our operating time for bilateral vasoepididymostomy is approximately 2 hours and 15 minutes, although in the earlier portion of this series it would have taken more than 5 hours. Our patients are requested to avoid strong physical activity for 4 weeks postoperatively and to remain at rest at home for the first week. We do not request them to begin having sperm counts until 3 months postoperatively, and then we request sperm tests approximately every 3 months thereafter.
General Information and Demographics
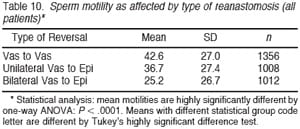
Table 1 summarizes the types of procedures performed for vasectomy reversal during each of the 3 previous decades. From 1975 to 1984, only 14.6% of vasectomy reversals required bilateral vasoepididymostomy. However, in the subsequent decade from 1985 to 1994, a total of 51.9% of the reversals required vasoepididymostomy, and over the entire 3 decades, 29.2% required bilateral vasoepididymostomy. The incidence of bilateral vasovasostomy declined from 58.4% in the first decade to only 20.2% of the cases in the last decade. Thus in the last 2 decades, only 22% of our vasectomy reversals involved bilateral vasovasostomy, and 78% required vasoepididymostomy on either one or both sides. What we observed is that the incidence of epididymal blowouts seen in our vasectomy reversal population have increased over the last 30 years as the popular techniques for vasectomy have emphasized more and more a tight, nonleaking seal of the distal vas.
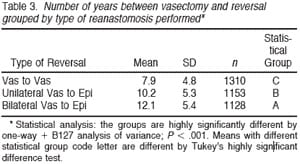
Table 2 summarizes the relation between the number of years since vasectomy and the type of anastomosis required, ie, bilateral vasovasostomy, unilateral vasoepididymostomy, or bilateral vasoepididymostomy. As the number of years between the vasectomy and the reversal increased, the percentage of patients undergoing simple bilateral vasovasostomy decreased dramatically as the number of patients requiring bilateral vasoepididymostomy increased. When the time since vasectomy was over 15 years, only 17.8% of patients underwent bilateral vasovasostomy and 47.9% underwent bilateral vasoepididymostomy. The longer of duration of time since vasectomy the greater the chance of finding no sperm in the vas fluid, epididymal blowouts, and, under our criteria, the greater the chance of requiring vasoepididymostomy (Table 3).
Outcome of Reversal as Assessed by Semen Analyses
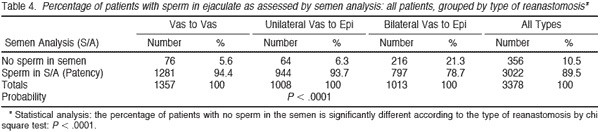
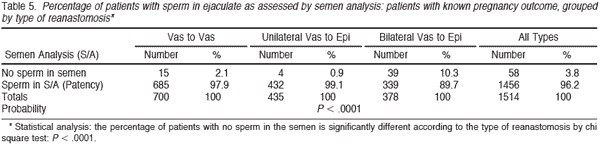
Of the 3904 patients with records available, 526 (13.5%) did not provide any postoperative semen analyses. Thus we had follow-up for semen analyses on 3378 (86.5%) of the patients. Table 4 summarizes the patency results for sperm in the ejaculate for all 3378 patients for whom these data were available, grouped by the type of reanastomosis. A small number of early patients (already reported in our 1977 paper), undergoing vasovasostomy in the early years had no sperm in the vas fluid, and by the criteria we established in 1977 would have now undergone vasoepididymostomy rather than vasovasostomy. The incidence of patency of sperm was 95% for vasovasostomy, 94% for unilateral vasoepididymostomy, and 79% for bilateral vasoepididymostomy. The overall patency rate was 90%, and much higher when at least one side required only vasovasostomy than when both sides required vasoepididymostomy because of absence of sperm in the vas fluid. Table 5 demonstrates the percentage of patients with sperm in the ejaculate (patency) who had a known pregnancy outcome (ie, whether the wife eventually became pregnant or did not become pregnant) grouped by type of reanastomosis (1514 patients). With this group of patients, of whom we have the best followup information, only 2.1% of those undergoing bilateral vasovasostomy failed to have sperm in the ejaculate, a 98% patency rate. For patients undergoing unilateral vasovasostomy with unilateral vasoepididymostomy, 99.1% had sperm (patency) in the ejaculate. Thus of a total of 1136 patients with vasovasostomy on one or both sides, only 19 (1.7%) failed to have sperm in the ejaculate postoperatively. Among patients undergoing bilateral vasoepididymostomy, almost 90% (89.7%) had sperm in the ejaculate postoperatively. Thus with this approach, performing vasovasostomy when there is sperm in the vas fluid and vasoepididymostomy when there is no sperm in the vas fluid, an overall patency rate of 96.2% was observed.
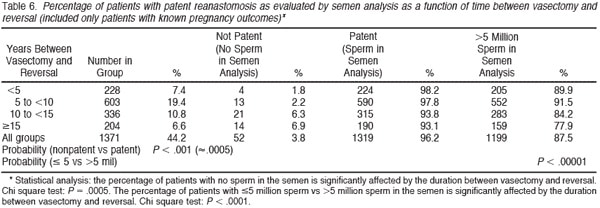
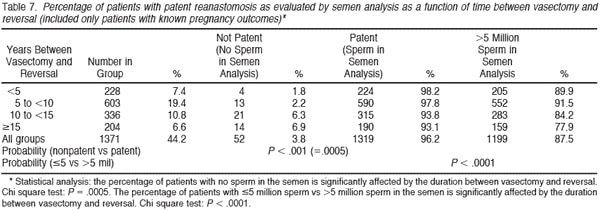
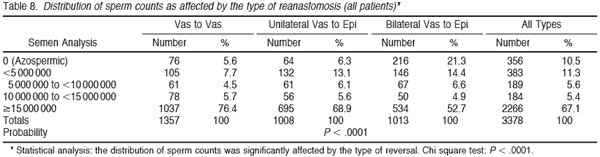
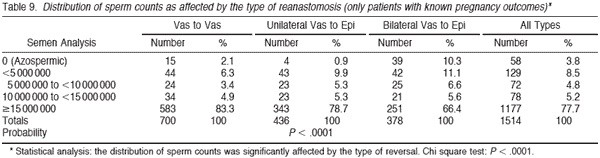
Tables 6 and 7 summarize the percentage of patients with sperm patency (and those with greater than 5 million sperm/mL) in the postoperative semen analysis according to the duration of time since vasectomy. The longer the duration of time since vasectomy, the greater the risk of nonpatency. If the vasectomy was less than 10 years earlier, approximately 95% of patients had sperm patency. However, if the vasectomy was more than 15 years earlier, 82% of patients had sperm patency postoperatively (P , .0001). Tables 8 and 9 demonstrate that approximately 75% of patients have greater than 15 million sperm/mL postoperatively. Once again it is clear that the semen analysis reveals a higher sperm count in men undergoing bilateral vasovasostomy than in men who require bilateral vasoepididymostomy. Table 10 in a similar fashion reveals that mean sperm motility percentage is higher for patients with bilateral vasovasostomy than patients with bilateral vasoepididymostomy. The percentage motility with bilateral vasoepididymostomy (despite a wide standard deviation), is almost half that resulting from bilateral vasovasostomy.
Outcome of Vasectomy Reversal as Assessed by Pregnancy in the Female Partner
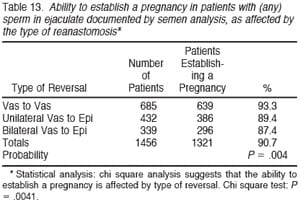
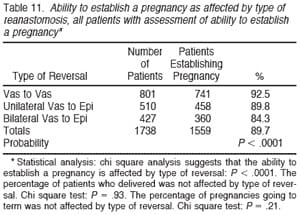
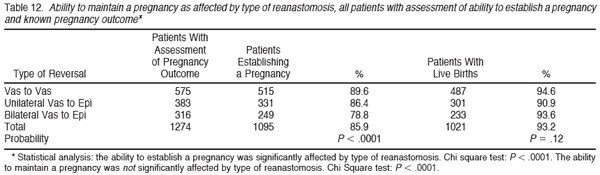
Table 11 demonstrates that the overall pregnancy rate, among 1738 patients on whom data are available, was 89.7%. There was a small decline in pregnancy rate for bilateral vasoepididymostomy (84.3%) compared with bilateral vasovasostomy (92.5%). Of the 1455 documented births, there were 731 boys (50.2%), and 724 girls (49.8%). There was no significant difference in boy:girl ratio according to the type of reversal required. The documented miscarriage rate (240 cases) for 2111 pregnancies (there was an average of 1.36 pregnancies in couples that established a pregnancy) was 15.4%. Table 12 subtracts out those pregnancies for which we could not document on follow-up whether birth occurred. Thus, in those patients where we could assess pregnancy outcome, 93.7% delivered, and there was no difference related to type of anastomosis.
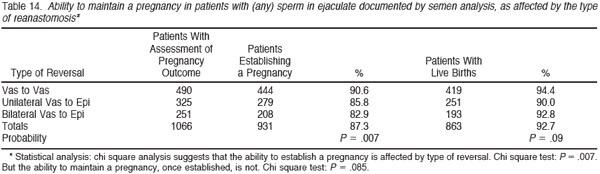
Table 13 summarizes the pregnancy rate according to type of anastomosis only for patients who had documented sperm patency. Of 1456 patients with sperm patency on whom adequate follow-up was available, 1321 were able to impregnate their wives, for an overall pregnancy rate in patent cases of 90.7%. The pregnancy rate was 93.3% for patients with a patent anastomosis after vasovasostomy and 87.3% for patients with a patent anastomosis after bilateral vasoepididymostomy. Table 14 again subtracts out those pregnancies for which we could not document whether or not live birth occurred, and 92.7% delivered.
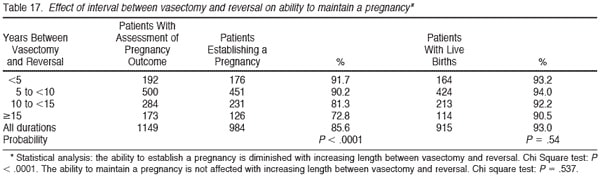
Table 15 summarizes the pregnancy rate according to the sperm count postoperatively. There is remarkably little difference in pregnancy rate related to postoperative sperm count. Once the sperm count is greater than 5 million/ mL there is no difference in pregnancy for those with high or low sperm counts. Table 16 demonstrates that the pregnancy rate goes down in proportion to the increase in duration of time since the vasectomy. When the time since vasectomy is less than 5 years, the pregnancy rate is 93.9%, whereas when the time since vasectomy is more than 15 years, the pregnancy rate is 79.8%. The overall pregnancy rate is 89.5% summing together all groups. Table 17 again subtracts out those pregnancies for which we could not document whether or not live birth occurred. For all durations of time since vasectomy, similarly about 93% delivered. Thus the pregnancy rate is still very high for patients with long duration of time since vasectomy, but nonetheless lower than when there is a shorter duration of time since vasectomy.
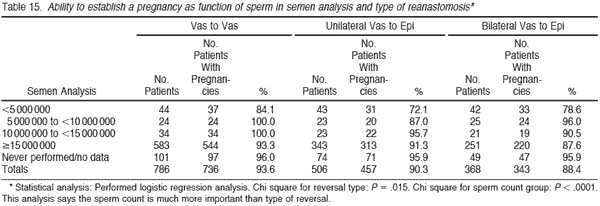
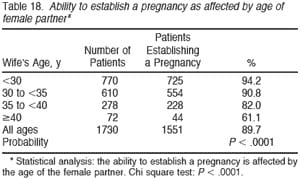
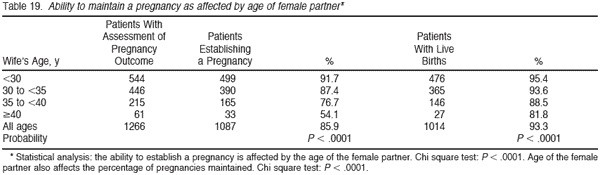
Table 18 summarizes the overall pregnancy rate for vasectomy reversal according to the age of the wife. Of all the factors analyzed, type of anastomosis, duration of time since vasectomy, and postoperative sperm count, the 1 factor that had the most significant impact on pregnancy rate was the age of the wife. When the wife was under 30 years of age, 94.2% of vasectomy reversals resulted in a pregnancy. When the wife was in her late 30s, 82% resulted in a pregnancy. However, when the wife was 40 or older, only 61.1% of the reversals yielded a pregnancy. In view of current in vitro fertilization (IVF) statistics, it is amazing that over 60% of women 40 or older would become spontaneously pregnant after a successful vasectomy reversal when IVF pregnancy rates for that group resulting in a delivery are normally less than 20% per cycle. Table 19 again subtracts out those pregnancies for which we could not document outcome, and the results are similar.
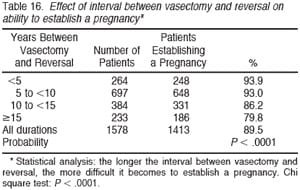
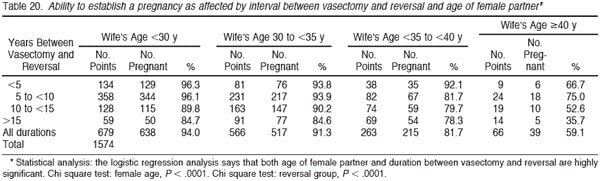
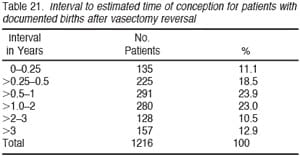
Table 20 breaks out the pregnancy rate according to the time since vasectomy as well as the wife’s age. Logistic regression analysis indicates that both the age of the female partner as well as the duration of time since vasectomy have significant impacts on the pregnancy rate, although the age of the wife is most critical. When the vasectomy was over 15 years earlier, and the wife was under age 30, still 84.7% achieved pregnancy. However, when the vasectomy was over 15 years earlier and the wife was 40 years of age or older, only 35.7% achieved pregnancy. Table 21 shows that 23.3% of all the pregnancies did not occur until more than 2 years after vasectomy reversal. Thus, inadequate follow-up is likely to underestimate ultimate pregnancy rate by a very large margin.
Perhaps the most controversial issue is whether actually to perform vasectomy reversal, rather than sperm retrieval with ICSI. Although we were the first to develop the concept of sperm retrieval plus ICSI for failed vasectomy reversal, the high spontaneous pregnancy rates achievable with microsurgical reversal of vasectomy do not support the preference of some centers for sperm retrieval plus ICSI over vasectomy reversal (Silber et al, 1994, 1995a,b). Perhaps the most urgent argument for going right to sperm retrieval plus ICSI occurs in cases where the wife is older. Yet IVF pregnancy rates when the wife is 40 years or older are seldom greater than 20%, and usually lower than that. By contrast, spontaneous pregnancy rates in women 40 years or older, married to men who have undergone a patent vasectomy reversal, is over 60%. The probable explanation for this discrepancy is that IVF represents just 1 cycle and for older women not that many eggs are obtained, whereas with vasectomy reversal the husband is inseminating his wife every month that she ovulates. So her chance of pregnancy per month might be only 4% or 5% but her overall chance of becoming pregnant after 2 or 3 years is still very good.
Thus, if the choice is being made between vasectomy reversal or sperm retrieval plus ICSI, whether the wife is younger or older, the observed data seemed to argue in favor of vasectomy reversal. It is clear that intravasal azoospermia, ie, the absence of sperm in the vas fluid, does not have a dramatic affect on the results so long as vasoepididymostomy is performed. The results of bilateral vasoepididymostomy were only marginally less favorable than the results with bilateral vasovasostomy. Thus if the surgeon is extremely competent with microsurgery, there is no good argument for not doing vasoepididymostomy. With this approach, the duration of time since vasectomy will only have a marginal effect on the success rate. Nonetheless, because the patency rate with vasectomy reversal is not 100%, it would still appear wise to freeze sperm retrieved at the time of surgery.
We have no way of knowing whether varicocelectomy would have been appropriate at the time of the vasectomy reversal, other than to say that, without doing a varicocelectomy on these patients, our results were still extremely favorable. That would argue against the benefit of varicocelectomy in this group of patients. This is consistent with data we have previously reported on varicocelectomy and vasectomy reversal (Silber, 1989c, 2000, 2001).
The controversial issue of sperm granuloma and openended vasectomy must now be addressed. Our current data reveal that from the years of 1975 to 1984 there was a much higher incidence of finding sperm in the vas fluid than in the subsequent 2 decades. There was also a higher incidence of sperm granuloma at the vasectomy site noted in our 1977 series than in the last several decades because urologists are in general much more fastidious now about sealing the testicular cut end of the vas, either with cautery or clips, so as to consciously avoid leakage of sperm at the vasectomy site. That high incidence of sperm granuloma at the vasectomy site in the 1970s accounts for the very high success rates we achieved with vasovasostomy only in our early reports in 1975 through 1978. Indeed the high success rate reported in earlier series that used a vasovasostomy exclusively (indeed that insisted upon vasovasostomy exclusively) was probably related the looser vasectomy seal in the decades preceding the 1970s and 1980s (Owen and Capila, 1984; Belker et al, 1991). In one such series gathered from 5 different surgeons, only 12 patients out of 1469 had undergone vasoepididymostomy, and only 83 (5.7%) patients out of that total 1469 did not have sperm in the vas fluid at the time of surgery. In our series, 29.2% had no sperm in the vas fluid on either side, and only 40.5% had sperm in the vas fluid on both sides. In the first 10 years of this series, almost 60% of patients had sperm in the vas fluid on both sides, and in the last 20 years of this series, only a little over 20% had sperm in the vas fluid on both sides. Thus as vasectomy techniques have become more leak-proof, results depending solely upon vasovasostomy without the capability of performing vasoepididymostomy would have to decline.
For this reason in 1977, 1978, and 1979 we published papers recommending open-ended vasectomy, which intentionally allowed the formation of sperm granuloma at the vasectomy site (Silber, 1977a, 1978a; Shapiro and Silber, 1979; Contraceptive Technology Update, 1986). This suggestion was greeted with vitriolic condemnation, and yet very large studies have subsequently documented its safety and utility. The sperm granuloma is a favorable finding at the vasectomy site and indicates that (whether or not sperm is found in the vas fluid) vasovasostomy will be successful in almost all cases. The granuloma works by allowing sperm leakage to be reabsorbed within the microcanalilculi of the sperm granuloma, thus preventing secondary pressure buildup and damage to the epididymis.
That leads into the next controversy. Are sperm antibodies responsible for an apparent discrepancy between patency rate and pregnancy rate in vasectomy reversal patients? Or is partial obstruction the problem? In 1989 we demonstrated that preoperative sperm antibody titers, just like preoperative varicocele, had no affect on the pregnancy rate after vasovasostomy (Silber, 1989d). Interestingly, the presence or absence of a sperm granuloma has no impact on the incidence of finding sperm antibodies postvasectomy. This is because whether there is leakage at the vasectomy site or leakage from pressure buildup in the epididymis, one way or another there is a high incidence of sperm antibody formation after vasectomy, and open-ended vasectomy does not increase that incidence. However, pregnancy rates in this current study as well as our previous report in 1989 argue strongly against any major negative impact of sperm antibodies on fertility after vasectomy reversal (Newton, 1988; Flickinger et al, 1995; Carbone et al, 1998).
There have been controversial and confusingly contradictory studies on the possible effect of vasectomy and pressure buildup on spermatogenesis both in humans and animals. Our data would argue against any clinical significance even if there is some sort of subtle testicular impact from vasectomy. Furthermore, all of our quantitative testicle biopsies reported in 1989 on that series of 282 patients followed for 10 years showed no significant deterioration in quantitative spermatogenesis in the testicle biopsy (Jarrow et al, 1985; Flickinger et al, 1990; Schoysman, 1990; Peng et al, 2002). The high success rate obtained with meticulous microsurgical technique argues in favor of Thomas’ postulate that infertility after vasectomy reversal is related to partial blockage either at the vasovasostomy site or at the epididymis rather than sperm antibodies or some other obscure nonmechanical factors (Carbone et al, 1998).
There have been controversial and confusingly contradictory studies on the possible effect of vasectomy and pressure buildup on spermatogenesis both in humans and animals. Our data would argue against any clinical significance even if there is some sort of subtle testicular impact from vasectomy. Furthermore, all of our quantitative testicle biopsies reported in 1989 on that series of 282 patients followed for 10 years showed no significant deterioration in quantitative spermatogenesis in the testicle biopsy (Jarrow et al, 1985; Flickinger et al, 1990; Schoysman, 1990; Peng et al, 2002). The high success rate obtained with meticulous microsurgical technique argues in favor of Thomas’ postulate that infertility after vasectomy reversal is related to partial blockage either at the vasovasostomy site or at the epididymis rather than sperm antibodies or some other obscure nonmechanical factors (Carbone et al, 1998).
Our data show that sperm count itself is not critical to pregnancy rate. In normal fertile populations (eg, men undergoing vasectomy after having all the children they want), there is normally a wide variation from low sperm count to high sperm count. For this reason, the sperm count alone after successful vasectomy reversal does not relate to pregnancy rate, but rather to the intrinsic sperm production rate in that man’s testes, which is not any different then than when he presented as a fertile young man prior to his vasectomy (Silber, 1989a,b; Paick et al, 2000). Therefore sperm count after vasectomy reversal in itself has no impact, whether high or low, on pregnancy rate so long as an anatomically meticulous anastomosis had been performed. We could speculate that low pregnancy rates in some reports after patent vasectomy reversal, whether vasovasostomy or vasoepididymostomy, are related either to the difficulty of obtaining accurate postoperative follow- up information over a long period of time or to partial obstruction with delayed sperm transport, sperm senescence, and subsequently poor fertilization. We reported in the late 1970s that in men who have had a reversal with conventional techniques with severe oligospermia postoperatively (less than 5 million sperm/mL and poor motility), a reoperation with a microsurgical approach resulted in an increase in the sperm count and subsequent pregnancy in the wife. However, the low sperm count itself was not the problem. It just correlates with what would be predicted to be the postoperative sperm count with the quantitative testicle biopsy. But, it is a problem if it results from a partial obstruction (Silber, 1989a).
There has been a suggestion that even in the face of intravasal azoospermia vasovasostomy can result in successful patency of sperm in the ejaculate without having to resort to vasoepididymostomy. There were only 5 such cases reported in the excellent series of Scheynkin and colleagues (Scheynkin et al, 2000). Of those 5 cases with sperm in the ejaculate after vasovasostomy despite intravasal azoospermia, 3 had crystal clear fluid (which we have always demonstrated is a good prognostic sign and does not indicate going to the epididymis) and 2 had no fluid whatsoever. No fluid whatsoever is usually caused by a sperm granuloma resulting in a so-called ‘‘dry vas.’’ There is absolutely no pressure buildup and therefore no accumulation of fluid, but we know these patients always have successful results after vasovasostomy. The point is that when there is vas fluid that is not clear, and this fluid contains no sperm, that is an indication of irreversible epididymal damage. For such cases, vasoepididymostomy is warranted, and many studies have shown that the epididymal damage this represents is not reversed by vasovasostomy (Flickinger et al, 1993; Srivastava et al, 2000). For that reason, and with further substantiation by the data in this paper, we still recommend vasoepididymostomy when the vas fluid is not clear and contains no sperm. These concepts were postulated in the review article one of us (S.J.S.) wrote for the Journal of Andrology in 1980, the first year of the journal, based on our early data in the 1970s. The 3-decade follow-up in this report, after more than 4000 cases, appears to support all of those principles originally formulated in that first volume of the Journal of Andrology.
Acknowledgments: The authors thank Lee Cohen-Gould and Becky Smith of the Electron Microscopy Core at Weill Medical College for assistance in preparing the electron micrographs and Ellen Kutner and Sharon Fuller for assistance with manuscript preparation. They also thank Dr. Michael DeRosa, Kathy Lenahan, and Dr. Jorge Pineda for their clinical assistance.
References
- Belker A. Technical aids for vasovasostomy. Urology 1982;20:635–637.
- Belker AM, Thomas AJ Jr, Fuchs EF, Konnak JW, Charlip ID. Results of 1,469 microsurgical vasectomy reversals by the vasovasostomy study group. J Urol. 1991;145:505–511.
- Carbone DJ Jr, Shah A, Thomas AJ Jr, Agarwal A. Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827–830.
- Chiang HS. Clinical study of vasectomy reversal: results of 60 single surgeon cases in Taiwan. J Formos Med Assoc. 1996;95:866–869.
- Derrick FC Jr, Frencilli FJ. Experiences with reversible vas device. J Urol. 1974;111:523–524.
- Derrick FC Jr, Yarbrough W, D’Agostino J. Vasovasostomy: results of a questionnaire of the members of the American Urological Association. J Urol. 1973;110:556–557.
- Donovan JF Jr. Microscopic vasovasostomy: current practice and future trends. Microsurgery. 1995;16:325–332.
- Fallon B, Miller RK, Gerber WL. Nonmicroscopic vasovasostomy. J Urol. 1981;126:361–362.
- Fenster H, McLoughlin MG. Vasovasostomy—is the microscope necessary? Urology. 1981;18:60–64.
- Flickinger CJ, Herr JC, Calores SD, Sisak JR, Howards SS. Inflammatory changes in the epididymis in the Lewis rat.Biol Rep. 1990;43:345.
- Flickinger CJ, Herr JC, Sisak JR, Howards SS. Ultrastructure of epididymal interstitial reactions following vasectomy and vasovasostomy. Anat Rec. 1993;235:61–73.
- Flickinger CJ, Howards SS, Bush LA, Baker LA, Herr JC. Antisperm autoantibody responses to vasectomy and vasovasostomy in Fischer and Lewis rats. J Rep Immun. 1995;28:137–157.
- Fox M. Vasectomy reversal—microsurgery for best results. Br J Urol. 1994;73:449–453.
- Fox M. Easing the technical difficulty of microscopic vasectomy reversal. Br J Urol. 1996;78:462–463.
- Fox M. Failed vasectomy reversal: is a further attempt worthwhile using microsurgery. Eur Urol. 1997;31:436–440.
- Friend DS, Galle J, Silber SJ. Fine structure of human sperm, vas deferens epithelium, and testicular biopsy specimens at the time of vasectomy reversal. Anat Rec. 1976;184:584.
- Fuchs EF, Burt RA. Vasectomy reversal performed 15 years or more after vasectomy: correlation of pregnancy outcome with partner age and with pregnancy results of in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2002;77:516–519.
- Huang HC, Chaise ML, Huang ST, Tsuei KH, Lai RH, Chang PL. Microsurgical vasectomy reversal: 10 years experience in a single institute. Chang Gung Med J. 2002;25:453–456.
- Inaba Y, Fujisaw M, Okada H, Arakawa S, Kamidono S. Clinical outcome for microsurgery for obstructive azoospermia.Int J Urol. 1999;6:139– 144.
- Jarrow JP, Budin R, Dym M, Zirkin BR, Noren S, Marshall FF. Quantitative pathologic changes in the human testis after vasectomy: a controlled study. NEJM. 1985;313:1256–1260.
- Jokelaine OS, Rintala E, Koskimies AI, Rannikko S. Vasovasostomy: a 15 year experience. Scand J Urol Nephrol. 2001;35:132–135.
- Lykins LE, Witherington R. Splinted vasovasostomy. Urology. 1978;11: 260–261.
- MacDonald GR, Edson M. Stented vasovasostomy. Urology. 1976;7: 200–201.
- Matsuda T, Horii Y, Muguruma K, Komatz Y, Yoshida O. Microsurgical epididymal vasovasostomy for obstructive azoospermia: factors affecting postoperative fertility. Eur Urol. 1994;26:322–326.
- McDonald SW. Vasectomy review: sequeli in the human epididymis inductis deferens. Clin Anat. 1996;9:337–342.
- Middleton RG, Smith JA, Moore MH, Urry RL. A 15 year follow up of nonmicrosurgical technique for vasovasostomy. J Urol. 1987;137: 886–887.
- Newton RA. IGG antisperm antibodies attach to sperm do not correlate with infertility following vasovasostomy.Microsurgery. 1988;9:278– 280.
- O’Connor VP. Anastomosis of the vas deferens after purposeful division for sterility. J Urol. 1948;59:299.
- Open-ended vasectomy prevents pain, may enhance reversibility. Contraceptive Technology Update. 1986;7:51–52.
- Owen ER. Microsurgical vasovasostomy: a reliable vasectomy reversal. Austral N Z J Surg. 1977;47:305.
- Owen E, Capila H. Vasectomy reversal: review of 475 microsurgical vasovasostomies. Med J Aust. 1984;140:398–400.
- Paick JS, Hong SK, Yun JM, Kim SW. Microsurgical singular tubular epididymal vasovasostomy: assessment in the era of intracytoplasmic sperm injection. Fertil Steril. 2000;74:920–924.
- Peng B, Zhang RD, Dai XS, Deng XZ, Wan Y, Yang ZW. Quantitative (stereological) of the study of the effects of the vasectomy on spermatogenesis in Rhesus monkeys (Macaca mulatta). Reproduction. 2002;124:847–856.
- Redman JF. Clinical experience with vasovasostomy utilizing observable intravasal stent. Urology. 1982;20:59–61.
- Rothman I, Berger RE, Cummings P, Jessen J, Muller CH, Chapman W. Randomized clinical trial of an absorbable stent for vasectomy reversal. J Urol. 1997;157:1697–1700.
- Rowland RG, Nanninga JB, Vincent J, O’Connor VJ. Improved results in vasovasostomies using internal plain cat gut stents. Urology. 1977; 10:260–262.
- Scheynkin YR, Chen ME, Goldstein M. Intravasal azoospermia: a surgical dilemma. Br J Urol Int. 2000;85:1089–1092.
- Schoysman R. Delay of appearance of spermatozoa in the ejaculate after vasoepididymostomy and vasovasostomy.Acta Eur Fertil. 1990;21: 125–131.
- Schrepferman CG, Carson MR, Sparks AET, Sandlow JI. Need for sperm retrieval and cryopreservation at vasectomy reversal. J Urol. 2001; 166:1787–1789.
- Shapiro EI, Silber SJ. Open-ended vasectomies, sperm granuloma, and post-vasectomy orchialgia. Fertil Steril. 1979;32:546–550.
- Shessel FS, Lynne CM, Politano VA. Use of exteriorized stents in vasovasostomy. Urology. 1981;17:163–165.
- Silber SJ. Microsurgical and clinical urology. Urology. 1975;6:150–153.
- Silber SJ. Microscopic technique for reversal of vasectomy. Surg Gynecol Obstet. 1976;143:630.
- Silber SJ. Perfect anatomical reconstruction of vas deferens with a new microscopic surgical technique. Fertil Steril. 1977a;28:72–77.
- Silber SJ. Microscopic vasectomy reversal. Fertil Steril. 1977b;28:1191– 1202.
- Silber SJ. Sperm granuloma and reversibility of vasectomy. Lancet. 1977; 17:588–589.
- Silber SJ. Vasectomy and vasectomy reversal. Fertil Steril. 1978a;29: 125–140.
- Silber SJ. Microscopic vasoepididymostomy: Specific microanastomosis to the epididymal tubule. Fertil Steril. 1978b;30:565–571.
- Silber SJ. Vasectomy and its microsurgical reversal. Urol N Am. 1978c; 5:573–584.
- Silber SJ. Epididymal extravasation following vasectomy as a cause for failure of vasectomy reversal. Fertil Steril. 1979;31:309–315.
- Silber SJ. Reversal of vasectomy and the treatment of male infertility. J Androl. 1980a;1:261–268.
- Silber SJ. Vasoepididymostomy to the head of the epididymis: recovery of normal spermatozoa motility. Fertil Steril. 1980b;34:149–153.
- Silber SJ. Reproductive infertility microsurgery in the male and female. Baltimore, Md: Williams & Wilkins; 1984.
- Silber SJ. Pregnancy caused by sperm from vasa efferentia. Fertil Steril. 1988;49:375–379.
- Silber SJ. Results of microsurgical vasoepididymostomy: role of epididymis in sperm maturation. Hum Rep. 1989a;4:298–303.
- Silber SJ. apparent fertility from human spermatozoa from the Caput epididymis. J Urol. 1989b;10:263–269.
- Silber SJ. Pregnancy after vasovasostomy for vasectomy reversal: a study of factors affecting long term return of fertility in 282 patients followed for 10 years. Hum Rep. 1989c;4:318–322.
- Silber SJ. The relationship of abnormal semen parameters to male fertility. Hum Rep. 1989d;4:947–953.
- Silber SJ. Evaluation and treatment of male infertility. Clin Obstet Gynecol. 2000;43:854–888.
- Silber SJ. The varicocele dilemma. Hum Rep Update. 2001;7:70–77.
- Silber SJ, Nagy ZP, Liu J, Godoy H, Devroey P, VanSteirteghem AC. Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Hum Rep. 1994;9:1705–1709.
- Silber SJ, Nagy Z, Liu J, Tournaye H, Lissens W, Ferec C, Liebares I, Devroey P, VanSterteghem AC. The use of epididymal and testicular sperm for ICSI: the genetic implication for male infertility. Hum Rep. 1995a;10:1031–2043.
- Silber SJ, VanSterteghem AC, Liu J, Nagy Z, Tournaye H, Devroey P. High fertilization in pregnancy rate after intracytoplasmic sperm injection with spermatozoa obtained from testicle biopsy. Hum Rep. 1995b;10:148–152.
- Srivastava S, Ansari AS, Lohiya NK. Ultrastructure of langur monkey epididymis prior to and following vasectomy and vasovasostomy. Eur J Morph. 2000;38:24–33.
- Witt MA, Heron S, Lipschultz LI. The post vasectomy length of the testicular vaso remnant: A predictor of surgical outcome in microscopic vasectomy reversal. J Urol. 1994;151:892–894.
- Yamamoto M, Hibi H, Yokol K, Mishima A, Katsuno S. Surgical outcome of microscopic vasectomy reversal: an analysis of 30 cases. Nagoya J Med Sci. 1997;60:37–42.