1Claus Yding Andersen, 2Sherman J. Silber, 3Stinne Holm Berghold, 4Jan Stener Jorgensen, 5Erik Ernst
1 Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, Faculty of Health Science, University of Copenhagen, Copenhagen, Denmark; 2 Infertility Center of St. Louis at St. Luke’s Hospital, 224 South Woods Mill Road, Suite 730, St. Louis, MO 63017 USA, (314) 576–1400; 3The Research Unit for General Practice, University of Southern Denmark, Odense, Denmark; 4Department of Ghynecology and Obstetrics, University of Odense, Odense, Denmark; 5Department of Gynecology and Obstetrics, Aarhus University Hospital, Aarhus, Denmark
Reproductive BioMedicine Online, March, 2012

Dr. Sherman Silber, a renowned pioneer in microsurgery and infertility, is considered one of the world’s leading authorities on IVF, sperm retrieval, ICSI, vasectomy reversal, male infertility, Y chromosome, tubal ligation reversal, egg and embryo freezing, ovary transplantation, and the reproductive biological clock. He performed the world’s first microsurgical vasectomy reversal, as well as the first testicle transplant, in the 70’s and now in the current century, the world’s first ovary transplant. He was the first to develop the TESE and MESA techniques for retrieving testicular and epididymal sperm in azoospermic men. He headed the clinical MIT team that first mapped and sequenced the Y chromosome in infertile men and discovered the now famous DAZ gene for male fertility. His research includes also the study of reproduction and fertility in zoo animals and endangered species. Most recently he has perfected the preservation of fertility for cancer patients with ovarian freezing and transplantation and thereby figured out how to extend the reproductive biological clock of women. He has helped pioneer minimal ovarian stimulation to reduce IVF costs.
Abstract
These three case reports describe the long-term duration of function of ovarian cortical tissue grafts among patients in a university fertility preservation programme in Europe and in a private practice programme in the USA. One woman underwent sterilizing cancer treatment and had frozen ovarian tissue transplanted, and two women underwent fresh ovarian tissue transplants. The function of ovarian cortical strips has continued for more than 7 years in these three women, with the birth of eight healthy babies following a single graft per patient. In addition to these three cases, transplantation (repeatedly in some cases) of cryopreserved ovarian tissue has restored reproductive function to all other women in the study centres’ programmes for some years. The sustained longevity of function of the transplanted tissue suggests that it may also be possible to postpone the normal time of menopause or to alleviate its symptoms.
Introduction
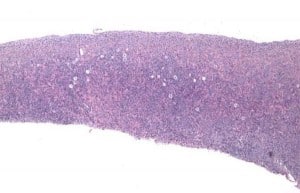
In recent years, there has been an increased focus on quality of life for the growing cohort of patients who survive a malignant disease in the early years (Lamer, DeCherney 2009). Fertility preservation is of high priority to many of these patients and planning for it may provide some emotional comfort and assurance while going through often harsh chemo- and/or radiation therapy, knowing they may regain menstrual cycles and create their own family once they have recovered from the disease. Not only will they be able possibly to have children after otherwise sterilizing treatment, but they may even be able to avoid hormone replacement therapy and not go through premature menopause (Wolff et al., 2009; Donnez et al., 2011). The first successful transplantation was achieved in 2004 (Donnez et al., 2004) and up until now transplantation of frozen–thawed ovarian tissue to menopausal women has resulted in the birth of a total of 18 healthy children worldwide (Donnez et al., 2004; Meirow et al., 2005; Silber et al., 2005; Demeestere et al. 2007; Silber and Gosden 2007: Andersen et al., 2008; Silber et al., 2008a; Silber et al., 2008b; Piver et al., 2009; Ernst et al., 2010; Sanchez-Serrano et al., 2010; Roux et al., 2010; Silber et al., 2010; Revel et al., 2011; Donnez et al., 2011; Dittrich et al., 2012; Silber 2012), and a total of 29 healthy children including fresh tissue homografts (Silber, 2012). The procedure is based on the fact that all of the primordial follicles of the ovary are located in the outer 1 mm of the fibrous cortex, and thus a simple skin graft like procedure can be employed (Figure 1). But the procedure is still considered experimental by many and the efficacy and the long-range durability of the transplants to sustain fertility have yet to be determined. The present paper describes long-term follow up of three women who have now given birth to second and third healthy children after having had frozen–thawed ovarian tissue transplanted more than 6 or 7 years ago, an indication of the robustness of this fertility preservation as originally reported in 2004 and 2005 (Donnez et al., 2004; Meirow et al., 2005; Silber et al., 2005; Demeestere et al., 2007; Andersen et al., 2008; Ernst et al., 2010).
Materials and methods
Patient one
Patient one was diagnosed with Ewing’s sarcoma at the age of 27 years. She initially received six series of vincristine/ifosfamide/doxorubicin/etoposide, after which the remaining tumour was removed surgically together with the two costae from which the tumour originated. Chemotherapy was continued with three series of vincristine/actinomycin D/ifosfamide. Prior to the cancer treatment, approximately one-third of the right ovary was excised and transported on ice for 5 hours before cryopreservation as already described (Schmidt et al., 2003). This was the patient’s only ovary as the other one had been previously removed due to a dermoid cyst. Following her cancer treatment, the patient’s menstruation stopped and post-menopausal concentrations of gonadotrophins and hot flushes developed.
Half of the retrieved and frozen ovarian tissue (i.e. six pieces of cortex approximately 5 ×5 mm in size) was transplanted to the remaining portion of ovary in December 2005, i.e. 21 months after cryopreservation, as previously described (Andersen et al.,2008; Ernst et al., 2010). After transplantation, her gonadotrophins gradually returned to premenopausal concentrations and her oestradiol concentrations increased. She conceived her first child following mild ovarian stimulation and delivered at week 39 a healthy girl weighing 3204 g by Caesarean section in February 2007. She then had a second spontaneous and uneventful pregnancy and had a successful vaginal delivery at week 42 in September 2008 of a healthy girl weighing 3828 g (Ernst et al., 2010), almost 3 years after her original transplant.
After having stopped breastfeeding her second child, the patient regained regular menstrual cycles once more, with 28-day intervals. In order to avoid further pregnancies, she used an intrauterine device for contraception. Concentrations of AMH were measured on two occasions following the second child and in both instances AMH concentrations were below the detection limit. A little less than 5 years after the initial transplantation, the intrauterine device was removed. Shortly thereafter, the patient conceived naturally again with no treatment and had a third uneventful pregnancy with delivery at week 41 of a third healthy boy weighing 4015 g in September 2011 almost 6 years after her first transplant of a few pieces of ovarian tissue.
Patient two
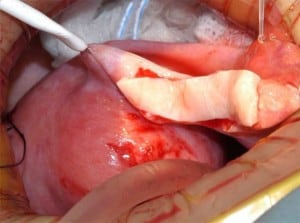
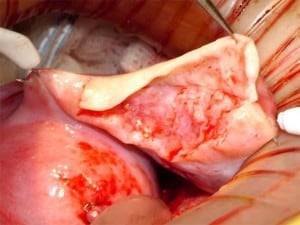
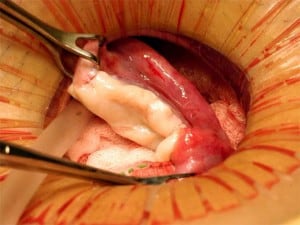
A 38-year-old woman underwent complete menopause at age 22, had an FSH concentration of 96.4 mIU/ml, an oestradiol of less than 15 pg/ml and no menstruation for the previous 16 years. Her identical twin sister had two healthy children and wished to have no more. One-half of one ovary was transplanted to her menopausal sister, by the technique already described (Silber et al., 2005; Silber, Gosden 2007; Silber et al., 2008a) in early 2005 (Figures 2–4).
The entire cortex of both menopausal ovaries was removed and revealed no follicles, verifying that any return of hormonal function or pregnancy would have to derive from the transplanted ovarian tissue. She began to menstruate 93 days after the ovarian tissue transplant and her day-3 FSH returned to normal 138 days after surgery. She became spontaneously pregnant 8 months post operation with no treatment and delivered a healthy baby girl vaginally at term (3250 g). She resumed menstruation even while breastfeeding at 23 months post operation and resumed normal regular menstrual periods until 41 months post operation and then once again became spontaneously pregnant with no treatment, now at age 42 years. She delivered her second healthy baby (a boy weighing 3280 g), again at term. Menstruation resumed when she stopped breastfeeding at 60 months post operation and then she became spontaneously pregnant once more at age 45, 7 years post operation and delivered a healthy baby boy in February 2012, weighing 3827 g and measuring 53.34 cm. All three children were conceived from the same piece of ovary transplanted from her 38-year-old identical twin sister 7 years earlier.
Patient three
A 33-year-old woman became menopausal following a bone marrow transplant with total body irradiation from her identical twin sister 4 years earlier for acute lymphoblastic leukaemia, She resumed menstruation on day 83 post operation and her day-3 FSH dropped to 4.4 mIU/ml. She continued to have normal monthly menses until she became pregnant 6 months post operation, but miscarried at 8 weeks. She resumed normal menses again until she once more became pregnant at 17 months post operation, but again miscarried at 6 weeks. She then resumed normal menses again until 33 months post operation when she once more became spontaneously pregnant (third pregnancy from same slice of transplanted ovary) and delivered a healthy boy weighing 1825 g prematurely at 34 weeks, who is quite healthy and developing normally now at 2 years of age. She again resumed normal menses several months later until she became spontaneously pregnant again (fourth pregnancy from same ovarian slice) at 4.5 years post operation and delivered another healthy baby boy 34 weeks later, at over 5 years post operation, who is developing normally now at 5 months of age. Her ovary slice continues to function normally almost 6 years post operation and there is still a majority of the donated ovarian slices frozen in liquid nitrogen.
Results
These cases show that just a small number of frozen–thawed ovarian cortical strips [technical video] (less than a third of an ovary) are capable of sustaining follicular development and produce viable oocytes competent for producing offspring for at least 5–7 years and, of course, possibly longer as these ovaries are still functioning at the time of writing. All pregnancies except the first in patient one, which was achieved through IVF, were spontaneous conceptions with no treatment other than the tissue transplant. This is the first report to demonstrate a series of cases where a woman has given birth to two or three healthy singleton children with three or more pregnancies as a result of a single transplantation of a small piece of ovarian tissue, whether fresh or frozen.
Discussion
Collectively, the present results show that fertility preservation based on cryopreservation of ovarian tissue can be an effective method to maintain fertility for many years and may provide a couple with the opportunity to create their own family more robustly than had previously been supposed. One might argue that multiple successive orthotopic transplants might fail due to adhesions, but this has not been found to be the case in previous reports (Silber, Gosden 2007; Sanchez-Serrano et al., 2010; Silber et al., 2010). This new technique may not only provide a realistic hope for saving the fertility for young patients surviving cancer treatment that would otherwise render them sterile and menopausal, but also for expanding a woman’s reproductive and hormonal lifespan long past its current limits. Again, one might argue that there are negatives to delaying menopause, but many women would disagree.
The longevity of frozen–thawed tissue is normally dependent on the age of the woman at cryopreservation and the amount of tissue replaced, and moderate duration of function has been reported when only a fraction of one ovary is transplanted (Greve et al., 2012). If one entire ovary is excised for freezing, this often allows women to have two or three transplantations. If their ovary is removed in their twenties with a relatively good ovarian reserve, a few women have experienced menstrual cycles for more than 7 years with the tissue still at work (Greve et al., 2012; Silber, 2012). Up until now there has not been a single woman in whom the tissue has not resumed activity as far as is known, but when only a small amount of tissue is available the tissue may stop working within 1 year (Greve et al., 2012).
Furthermore, a number of these women have many more pieces of ovarian cortex still stored in liquid nitrogen. If the already-grafted tissue remains active for over 6–7 years, as seems to be the case, there are far greater implications than allowing cancer patients to conceive. One patient is now 34 years of age, one is 45 and one is 39. So with further transplantation of frozen tissue, they may experience ovarian function and menstrual cycles for as long as, or even longer than, most normal women.
However, some women of similar age and circumstances may experience a considerably shorter duration of the transplanted ovarian tissue. The reason for the women reported here having exceptionally long duration of the graft is currently unknown, but these cases illustrate what is possible to achieve and call for much more research to define the circumstances by which the primordial follicles survive freezing and transplantation. A focused effort to define these appropriate conditions is obviously needed at this point in time in order to make this a realistic option available to more women in the future.
Indeed, these patients offer an insight into a perhaps futuristic method of postponing menopause in order maintain fertility or to maintain circulating concentrations of sex hormones that may reduce sequalae from a predisposition of osteoporosis or other menopausal problems. Up to 50% of newborn girls in many Western societies can expect to live to 100 years of age (Christensen et al., 2009). Indeed, if this is the case some of these women may experience sequalae from being without circulating sex hormones for so long and although this is still a theoretical consideration some of these women may not wish to spend a full half of their lives in menopause. Removing a single ovary from a young woman will not reduce her fertility or hasten her age of menopause if it could be frozen and slices transplanted back at age 50. Alternatively, the women may use hormone replacement regimens that are well established and have been in use for many years. There is a small increased risk of breast cancer for women taking hormone replacement therapy and a similar risk may be present for women having ovarian tissue transplanted, but they will experience natural variations in sex hormones that potentially may protect them more efficiently from osteoporosis and other menopausal symptoms, but that remains to be determined.
So, although most women having ovarian tissue stored today have suffered from a malignant disease, this method may also become interesting for women without a malignant disease. In this case, there will be no risk of transmitting the original cancer back together with the ovarian disease, which at this point in time cannot be totally excluded even though the worldwide experience with transplanting frozen–thawed ovarian tissue from women with a former malignant diagnosis now approach 50 cases without any reports of relapse. Further, there are a number of reassuring reports on malignant diseases like breast cancer and Hodgkin’s disease (Kim et al., 2001; Meirow et al., 2008; Sánchez-Serrano et al., 2009; Azem et al., 2010; Rosendahl et al., 2011).
The majority of children derived from ovary tissue transplantation have been born following natural conception. This result strengthens the fact that transplantation to the remaining post-menopausal ovary provides a suitable environment to support follicular development and enable conception without assistance. Further, there is currently no sign of this procedure having any adverse effects on the children. However, when transplantation is performed to the remaining ovary it is impossible to know with certainty whether residual resident follicles have caused the pregnancy, unless the atrophic cortex has been completely removed, as was done in two of these three cases.
It is anticipated that these pregnancies had to be the results of transplanted tissue, as indeed a recent announcement from Spain has indicated, where a woman who had both ovaries excised later became pregnant from replacement with frozen–thawed ovarian tissue (Rada, 2012).
In conclusion, the present result supports cryopreservation of ovarian tissue as a valid method of fertility preservation and encourages further development of this technique to be used in a clinical setting in girls and woman facing gonadotoxic treatment and possibly to expand the reproductive and endocrine lifespan of women.
Acknowledgements
The financial support from the Danish Cancer Society (DP05112/ R2-A41–09-S2), The Danish Medical Research Council (271–07–0452; 09–072265), the Novo Nordic Foundation, Sophus Carl Emil Friis and wife Olga Doris Friis’ foundation, and the University Hospital of Copenhagen are gratefully acknowledged. The American patients were financed by a policy of free treatment for fertility in cancer patients at St. Luke’s Hospital in St. Louis, Missouri, USA.
References
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod 2008;23:2266–2272.
- Azem F, Hasson J, Ben-Yosef D, Kossoy N, Cohen T, Almog B, et al. Histologic evaluation of fresh human ovarian tissue before cryopreservation.Int J Gynecol Pathol. 2010;29:19–23
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist 2007;12:1437–1442.
- Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, van der Ven H, Montag M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation.Fertil Steril. 2012;97:387–90
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004;364:1405–1410.
- Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437050.
- Ernst E, Bergholdt S, Jorgensen JS, Andersen CA. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod 2010;25:1280–1281.
- Greve T, Schmidt KT, Kristensen SG, Ernst E, Yding Andersen C. Evaluation of the ovarian reserve in women transplanted with frozen and thawed ovarian cortical tissue. Fertil Steril (in press).
- Kim SS, Radford J, Harris M, Varley J, Rutherford AJ, Lieberman B, Shalet S, Gosden R. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod. 2001;16:2056–60.
- Lamar CA, Decherney AH. Fertility preservation: state of the science and future research directions. Fertil Steril. 2009;91:316–319.
- Meirow D, Levron J, Eldar-GevaT, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005;353:318–321.
- Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–13.
- Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. In: 25th Annual Meeting of ESHRE, 28 June = 1 July, 2009. Amsterdam, The Netherlands: Oxford University Press, Hum Reprod. 2009:i15.
- Rada AC. Spanish woman becomes pregnant through ovarian tissue transplantation, BMJ 2012;344:d8350 doi: 10.1136/bmj.d8350
- Revel A, Laufer N, Ben MA, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod 2011;26:1097–1103.
- Rosendahl M, Timmermans Wielenga V, Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, et al., Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011;95:2158–2161
- Roux C, Amiot C, Abnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril 2010;93:2413.
- Sánchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, Pellicer A. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril 2010;93:268.
- Schmidt KL, Ernst E, Byskov AG, Nyboe Andersen A, Andersen CY. Survival primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod 2003;18:2654–2659.
- Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, Crawford EC and Gosden RG. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med 2005;353:58–63.
- Silber SJ and Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med 2007;356:1382–1384.
- Silber SJ, Grudzinskas G and Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med 2008a;359:2617–2618.
- Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod 2008b;23:1531–1537.
- Silber S, Kagawa N, Kuwayama M, and Gosden, R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril 2010;94(6):2191–2196.
- Sánchez-Serrano M, Novella-Maestre E, Roselló-Sastre E, Camarasa N, Teruel J, Pellicer A. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Hum Reprod. 2009;24:2238–43.
- Wolff M von, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, Salle B, Sonmezer M, Yding Andersen C: Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy – a technique in its infancy but already successful in fertility preservation. Eur. J. Cancer 2009;45:1547– 1553.
Declaration: The authors report no financial or commercial conflicts of interest.
See also: