
By S.J. Silbera,b, N. Barbeya
aInfertility Center of St. Louis, St. Luke’s Hospital, 224 South Woods Mill Road, St. Louis, MO 63017, USA, bCenter for Reproductive Medicine, Women’s and Children’s Hospital, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
Biochimica et Biophysica Acta,, October 6, 2012
Download PDF version of this article
Abstract
Purpose. The purpose of this review is to summarize science-based new treatments for human reproductive failure and future developments. Results. First will be discussed popular but erroneous myths of current non-science based treatments. Then will be discussed new treatments and their scientific base, including ovary and egg freezing, and transplantation to preserve fertility in young women undergoing gonadotoxic chemotherapy and radiation for cancer; new perspectives on human epididymal sperm maturation based on a comparison between ICSI (intracytoplasmic sperm injection) with testis sperm versus epididymal sperm; simplifying IVF and reducing cost by more intelligent and milder ovarian stimulation; improving pregnancy rate in older women; searching the genome to find genes which control spermatogenesis and whose deletion or mutation causes spermatogenic failure; and human spermatogenic stem cell culture to treat azoospermia, and to preserve fertility in pre-pubertal boys undergoing cancer treatment. Conclusion. With stem cell biology and molecular understanding of reproductive failure, new therapies for previously untreatable infertility are currently on the near horizon. Conversely our clinical results with new therapeutic approaches are adding to our understanding of the basic science of reproduction. This article is part of a Special Issue entitled: Molecular Genetics of Human Reproductive Failure.
Introduction
The developed world is in the midst of a widespread infertility epidemic. Economies in Japan, the United States, southern Europe, and even China are threatened by a decreasing population of young people having to support an increasing population of elderly and retirees [1]. The most common reason to see a doctor in countries like India and China, seemingly plagued with overpopulation, is for infertility. Infertility clinics are popping up throughout the world in huge numbers [2]. But much of the treatment is empirical, based on poor to no scientific footing, and even lacks proper statistical evidence for effectiveness. In most instances infertility is idiopathic, and there is even poor understanding of why IVF does or does not work in many cases [2,3].
Current treatments and myths
Male factor
We know that sperm counts can range from zero to 500,000,000 or more per ml [4–7]. But we have no solid evidence for what is the threshold, if any, for the sperm count to exceed in order to allow the male to be fertile [4,5,7]. Yet billions of dollars are spent each year for dubious treatments to try to increase the man’s sperm count, ranging from empirically placing him on Clomid, testosterone, HCG (human chorionic gonadotropin), FSH (follicle stimulating hormone), aromatase inhibitors, nutritional supplements, varicocelectomy surgery, cold jock straps, abstinence until the time of ovulation, testosterone rebound, etc., all in a futile effort to raise a sperm count that cannot be raised and which possibly does not need to be raised anyway [4–9].
Now it is true that microsurgery can correct obstruction like for a vasectomy [10], and the very rare case of Kallmann Syndrome can be corrected with gonadotropin administration; and ICSI (intracytoplasmic sperm injection) with IVF and sperm retrieval are valid treatments for azoospermia or the most severe oligospermia. But for the most part, there is no scientific evidence for male infertility treatment [2,5,11].
Female infertility
It is clear to all that the major reason for the world’s growing infertility epidemic is that as women put off childbearing, their eggs die off and those that survive are of poor quality [12–15]. In her teen years a woman has a 0.2% chance of being infertile, and by her early twenties it is up to 2%. By her early thirties, it is up to 20% [2,16]. Most modern women today do not think of having a baby until their mid-thirties, and by then over 25% are infertile, simply because of the aging and the decline in number of their oocytes. This is clearly demonstrated by the high pregnancy rate using donor oocytes from young women placed into the uterus of older women [2,3,16]. Yet fertility physicians struggle to make a pathological entity diagnosis to explain the infertility, which in truth in most cases is just a normal physiologic response to oocyte aging [2,12,16].
What are some of the disastrous consequences of the empirical, non-science based treatment of these misdiagnoses? Let’s look at “endometriosis” first. Endometriosis simply means that there are small pieces of endometrial tissue located in the pelvic peritoneum or in the ovary, and there should be nothing surprising about this occurrence in view of the commonality of retrograde menstruation. Supposedly this ectopic endometrial tissue causes dysmenorrhea, although most periods are painful in most women to some degree anyway, and endometriosis is usually not the cause of these painful periods. But there has never been an intelligent explanation for why these usually very small amounts of ectopic endometrial tissue would cause infertility. Certainly the pregnancy rates using IVF for women with endometriosis are no lower than for those without endometriosis [17]. Most randomized controlled studies fail to show a difference in pregnancy rates in couples with endometriosis who have or have not been treated for it [17].
But what is the danger of either an unwarranted varicoelectomy in a male, or an ablation of endometriosis in a female? For varicocoelectomy, it is easy to accidentally reduce the blood supply to the testicle or to have lymphatic scrotal swelling, but most importantly, it delays the IVF treatment with ICSI until the female partner’s eggs are older and less fertile [9,18]. For ablation of ovarian endometriosis, laparoscopic stripping has been shown to reduce the ovarian reserve, even if performed by experienced surgeons [19]. So in an effort to cure the endometriosis, the female’s ovarian reserve is almost always reduced.
Another popular but non-scientifically based treatment for the human female involves PCOS (polycystic ovary syndrome). Metformin is given to almost every non-ovulating woman with irregular cycles under the assumption that PCOS is just another type of diabetes mellitus. So these women, who have no glucose intolerance are commonly given a drug (which has huge side effects) that causes glucose and potassium to go intracellular and rarely results in correction of the failure to ovulate [20].
Science based major new therapies and new horizons
Aside from IVF and ICSI, which are long-term standardly accepted treatments, the rest of this chapter will review new science based and future therapies Preserving fertility for women who wish to put off childbearing or who are about to undergo treatment with gonadotoxic drugs: using ovarian freezing and transplantation.
Until recently oocyte freezing had very poor to no success, and so ovary tissue slow freezing was the only method we could rely upon for preserving oocytes of patients with cancer prior to otherwise sterilizing cancer treatment [21]. However, now we also have a favorable option of retrieving oocytes after ovarian stimulation and egg retrieval, and using vitrification
for cryopreservation [13,22]. Nonetheless, ovarian tissue freezing and transplantation still has great advantages over egg freezing in that there does not need to be a delaying stimulation cycle prior to the cancer treatment. Furthermore, one cycle of ovarian stimulation and egg freezing does not assume success of pregnancy like an entire ovary would, and finally, transplanting ovarian tissue back not only restores fertility but also endocrine function.
Fresh series of identical twins with POF
The first successful fresh human ovary transplantation was reported between a pair of remarkable monozygotic twins discordant or premature ovarian failure (POF) using a cortical grafting technique [23]. The first successful human frozen ovary auto-grafts were reported around the same time with tissue cryopreserved for patients with cancer
prior to their sterilizing bone marrow transplants [24,25]. This followed similar results described in the sheep over a decade earlier [21]. The technique has subsequently been refined over a larger series of nine consecutive successful fresh ovary transplants in identi- cal twins (plus two fresh allotransplants to be treated separately), with resumption of normal hormonal cycling and menstruation in all cases, eventually leading to 14 pregnancies and 11 healthy babies born from the nine fresh identical twin recipients [14,26–28]. This unusual consecutive series of fresh ovary cortical transplants helped us also refine the techniques necessary for successful preservation of fertility for patients with cancer using ovarian tissue freezing, with 3 additional successful pregnancies from 3 frozen transplants. This unusual series also helped to establish a method for distinguishing between the egg loss from transplant ischemia versus the egg loss from cryopreservation. We now can report long-term follow-up (up to 8 years) of this original series of fresh transplants, and add to it our more recent experience with cryopreserved ovarian tissue. The results appear to be remarkably more robust than had originally been contemplated [15,29].
The first such twins case inquired about this possibility originally from researching an earlier testis transplant we had reported for anorchia [23,30,31]. From that point forward, patients in similar situations sought this treatment. The patients volunteered many reasons for preferring transplantation over conventional oocyte donation. Many of them had previous failures with donor oocyte IVF cycles, and did not want to go through more IVF. Some had the opportunity to donate an ovary at the same time as having surgery they required for other gynecologic problems (such as fibroids or cysts). All of them found the possibility of natural conception more attractive than IVF or egg donation. In most cases, the twins lived far apart (even in different countries) and the donors preferred to make a single visit for a one-time ovary donation, rather than go through multiple cycles of ovarian hyperstimulation. We knew when we began this series that there would be few clinical cases in the world like these that would warrant fresh ovarian transplantation. However, this series would allow us to learn how to more effectively freeze and transplant human ovarian tissue, which would have far reaching consequences and widespread application for preservation of fertility in cancer patients and for women who just need to delay childbearing for social reasons. Despite risks, the evidence does not support a deleterious effect of unilateral oophorectomy either on fertility or on age of menopause [32,33].
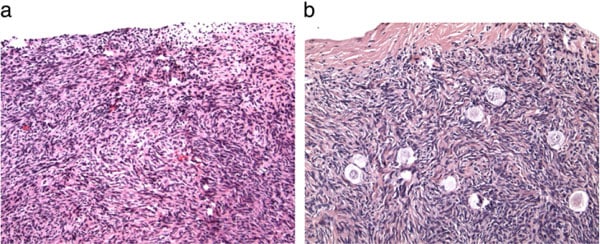
One entire ovary of the donor was removed and the cortex dissected away from the medulla. The cortex of the non-functioning recipient ovary was removed in entirety, and the donor cortex slices were transplanted onto the exposed recipient medulla using 9–0 nylon interrupted sutures. A tiny piece of spare tissue of the donor, as well as the entire resected atrophied ovarian cortex of the recipient were examined histologically in all cases (Fig. 1a and b). Micro-hematoma formation under the graft was avoided by micro-bipolar cautery and micropressure stitches of 9–0 nylon. Constant pulsatile irrigation with heparinized saline prevented adhesions (Fig. 2a–d). Only one-third of the ovarian cortex was grafted fresh and two-thirds were frozen.
Ovarian cryopreservation
All of our fresh clinical transplant studies involved cryopreservation of spare tissue for future thawed transplants. We also have frozen the ovarian tissue of 68 patients with cancer and 7 patients who simply want to delay childbearing. All of the frozen cases thus far transplanted back to the patient have utilized the slow freeze approach [21,34,35]. However, we now use vitrification exclusively for cryopreservation in humans because of our in vitro viability analysis studies as well as in vivo transplant studies in the bovine [14,36].
We developed this view by studying patients with cancer who were requesting fertility preservation by ovarian banking and who consented to an oocyte viability test and histologic review of a small sample (b10%) of their fresh or cryopreserved tissue. The goal of the in vitro study was to determine which method produced a higher cell survival rate. The high viability (92%) of oocytes in both control (fresh) specimens and vitrified specimens indicated that disaggregation per se had only caused minimal damage to this cell type [14]. Overall 2301 oocytes were examined from 16 specimens. Results within each of the three groups revealed no significant difference overall between fresh and vitrified tissue, but the viability of slow freeze-cryopreserved tissue was less than half that of vitrified tissue or controls (42%) (P b .01). Transmission electron microscopy also has been used to analyze ovarian tissue that had been either cryopreserved by slow freezing or vitrified by ultra-rapid freezing, showing vitrification to be superior [37]. Finally quantitative histologic study of primordial follicles in the bovine after vitrification and transplantation back to the cow two months later remarkably showed no follicle loss.
The basic science concept of vitrification, whether for eggs, embryos, or tissue, is to completely avoid any ice crystal formation by using a very high concentration of cryoprotectant and a very rapid rate (virtually “instant”) of cooling. This is quite different from classic slow freeze cooling which relies on a partial and very gradual removal of water from the cell by encouraging ice crystal formation preferentially on the outside of the cell, drawing the water out [36,38].
Cortical ovarian tissue transplant technique
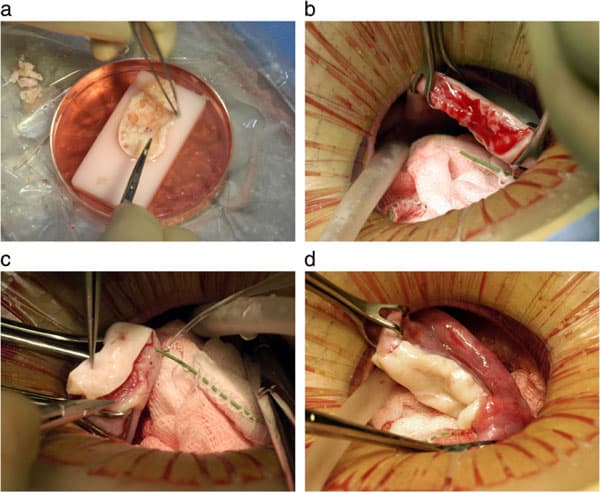
Under general anesthesia, one ovary is removed from the donor using laparoscopy or minilaparotomy. The whole ovary is transferred to a Petri dish for dissection. It was felt important to prepare a cortical tissue slice no thicker than ≅1.5 mm to facilitate rapid revascularization while keeping the tissue constantly irrigated with ice-cold Leibovitz L-15 medium (Fig. 2a). We now recommend a special Kitazato tissue slicer rather than dissecting by hand, in order to get a thinner slice. But for these initial fresh cases, the hand pared cortex was divided into several pieces of approximately equal size for grafting, one piece to each recipient ovary. The remaining two-thirds of the cortical tissue was cryopreserved [21,34,35,39,40]. The technique for transplanting thawed ovarian cortical tissue is no different than for fresh cortical tissue.
The recipient ovarian cortex resected via minilaparotomy to lay bare the medullary tissue (Fig. 2b); hemostasis is controlled with microbipolar forceps, and pulsatile irrigation with heparinized saline avoid adhesion formation or micro hematomas between donor and recipient tissues. Avoiding microhematoma formation beneath the graft may be the most important reason for minimal ischemic loss of oocyte viability. The tissue graft is attached using 9–0 interrupted sutures under an operating microscope (Fig. 2c). Very importantly, the medullar bed is also sutured to the under surface of the cortical graft with 9–0 sutures to maintain tight tissue approximation, again to avoid microhematoma formation under the cortical graft (Fig. 2d).
Intact whole ovary transplantation
Before we had long-term follow-up of the cortical tissue grafts, we had postulated, incorrectly we now believe, that we could lengthen graft survival and avoid ischemic loss of follicles by doing instead a whole intact ovary microvascular transplantation.
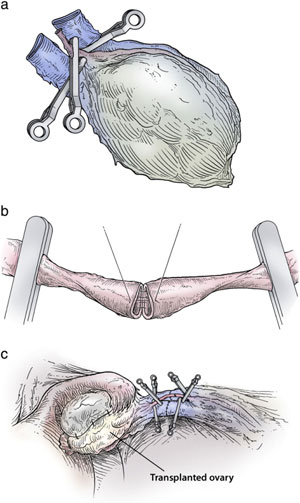
To transplant an intact whole ovary, the donor ovary is removed by clamping the infundibular pelvic ligament at its base in order to obtain maximum length. The veins (3–5 mm) are easily identified, but the ovarian artery (.3 mm) is often not grossly visible. The entire specimen is placed in Leibovitz medium at 4 °C and two veins and one artery were dissected and isolated under the operating microscope. The recipient’s infundibular pelvic ligament is clamped at the base and transected close to her non-functioning ovary. The donor’s ovarian veins are then anastomosed to the recipient’s with 9–0 nylon interrupted sutures, and the ovarian arteries are anastomosed with 10–0 nylon interrupted sutures (Fig. 3a–c). The original concept behind whole intact ovary microvascular transplantation was to avoid the supposed damage that was incorrectly attributed to cortical grafting [41]. However, current results seem to eliminate the need for whole ovary transplantation, as the much simpler fresh cortical ovarian tissue grafts have now been shown to have a very long duration of function.
Results of fresh and frozen ovarian transplantation
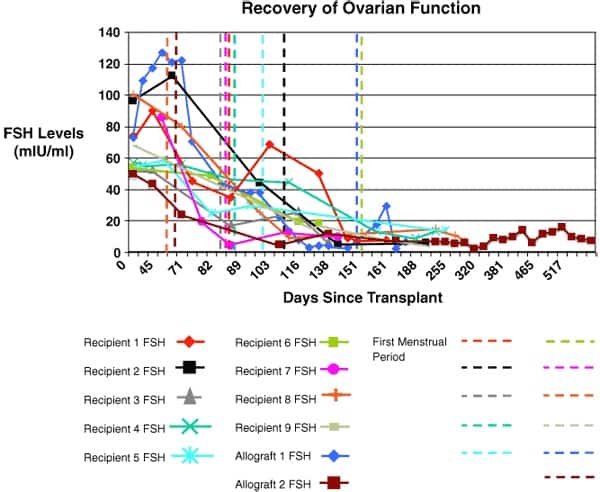
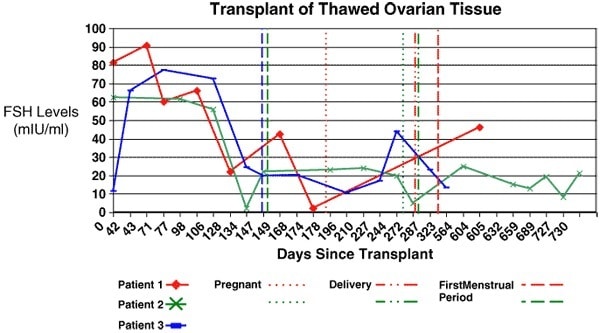
Results are summarized in Table 1 and in Figs. 4 and 5. All nine identical twin pairs underwent their orthotopic ovarian isotransplantation between April 2004 and April 2008. The recipients, for the most part, continued to cycle from two years in two patients whose donor had low ovarian reserve to over seven years in most cases. The two whose donor had low antral follicle counts (AFC) of less than 10, only functioned for 2 years. However, even these two cases had spare frozen cortical tissue that remains available for future transplants. Menstrual cycles began within 3 months, and Day 3 FSH levels returned to normal by 4.5 months and ovulation resumed then in all cases (Figs. 4 and 5). A total of 14 healthy babies resulted from the 12 ovary transplants, 11 from the 9 fresh transplants, and 3 from the three frozen transplants (Table 1).
One of our identical twin recipients became pregnant at 39 years of age (from a graft that was of course also 39 years of age), eight months after transplantation. She delivered a healthy baby girl at full-term and then conceived again at age 42 from the same graft, and delivered a healthy baby boy, again at full-term, 4 years after her transplant. Her ovary is still functioning to date after seven years, and she conceived again at age 45 with another healthy boy, more than seven years after her transplant. One case of ovary transplant was an identical twin whose POF (premature ovarian failure) was caused by a bone marrow transplant with pelvic irradiation for leukemia, with her identical twin sister being the donor. She became spontaneously pregnant 5 months after her fresh ovary transplant from her sister, but miscarried. Then at 1.5 years she became pregnant again and had a healthy baby, and at 5 years after the transplant, she became pregnant again, and had a second healthy baby. Over 6 years later, her original transplant is still functioning, and she still has 2/3 of an ovary that remains frozen. It does not appear from this or from the frozen cases that transplant ischemia should limit the duration of function or that pelvic radiation is incompatible with a healthy pregnancy.

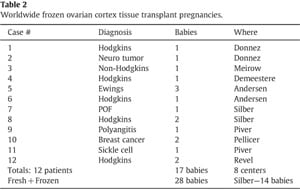
This newly favorable experience with ovarian cortex grafting is not limited just to our center [42]. Equally robust results are being experienced in Brussels, Paris, Spain, Denmark, and Israel (personal communication). Frozen ovarian grafts (even with the slow freeze technique) in Denmark are lasting over 5 years and many spontaneous pregnancies have been reported with no need for IVF or other ancillary treatment. At the time of this writing, 30 healthy babies have been born from ovarian tissue grafting fresh and frozen, and most involved no IVF, and resulted from just regular intercourse with no special treatment (Table 2).
Frozen cortical ovarian transplantation
The most common benefit of ovarian transplant is not the unusual cases of fresh grafting in identical twins but rather to protect the fertility and future endocrine function of young women undergoing cancer treatment [14,36,43–49]. Since 1996, we have frozen ovary tissue for 68 young women with cancer, or at risk for POF, of whom 16 had spare frozen tissue subjected to detailed viability testing before cryopreservation and after thaw. All 68 had histologic review by a variety of pathologists. Only one had ovarian metastasis, a young woman with widespread breast cancer metastasis throughout her entire body. Otherwise, none of our other 67 cases had any tumor cells in their ovary as judged by microscopical analysis of histological sections. Andersen has also noted a complete lack of ovarian metastasis, even in the majority of leukemia cases (Fig. 6) [50]. The reason for the remarkable absence of ovarian metastasis might possibly be due to the fibrous avascular nature of the ovarian cortex. In fact, the reason why fetal ovarian cords (which in the fetal male become seminiferous tubules) invade the fibrous cortex and become follicles, is that the dense fibrous tissue of the cortex (which in the fetal and adult testis is just tunica albuginea) is needed to suppress the resting follicles from developing all at once prematurely. By analogy, in the male, leukemic cells do indeed routinely lodge in the testis proper, but not ever in the tunica albuginea of the testis. That same phenomenon protects these ovarian cortical slices as well.
Genetics of non-cancer premature ovarian failure, and low ovarian reserve
Identical twins discordant for ovarian function present a true genetic puzzle [33]. The great majority of women enter menopause in their fifth or sixth decade of life, at an average of 51 years, but 1% undergo menopause quite prematurely, i.e. before 40 years of age [51–53]. Premature ovarian failure (POF) is assumed to have a genetic etiology and menopausal age normally is strongly heritable judging by the greater concordance between monozygotic (MZ) than dizygotic twins [54–57]. It was remarkable, therefore, to identify MZ twin pairs in which one sister had undergone menopause for unexplained reasons at a very early age from 14 to 22 years, whereas the other, was still fertile with naturally conceived children, as well as normal ovulatory cycles and ovarian reserve [23,26–28].
We have not yet taken advantage of the unique opportunity these twins offered for studying the genetic origin of ovarian reserve, but genomic DNA and lymphoblastoid cell lines were prepared and carefully stored for future genetic and epigenetic studies. Details of the obstetric records on the original chorionicity at birth of these identical twin sisters revealed 50% were monochorionic–monoamniotic, which was surprisingly high since the incidence of mono/mono is normally only ≈ 2% (P b 0.0005). It is clear that late splitting of the inner cell mass of the blastocyst, for whatever reason, predisposes otherwise identical twins to discordant germ cell deficiency [27,58,59].
Thus far the search for genes controlling ovarian reserve in the human has yielded meager results, the only modestly common candidate being the pre-mutation carrier status for fragile X (FMR1). The answer oddly enough may lie in a search for genes on the X-chromosome analogous to the search that has been conducted for genes on the Y-chromosome that control spermatogenesis (see below). The human Y-chromosome contains a huge concentration of amplicons and palin- dromes, which are very difficult to sequence [8]. These regions of long sequence identity with many multi-copy genes, would not have been sequenced with routine methods and most would have been missed in the sequencing without special methodology. Similar regions on the X chromosome that did not undergo the very specialized type of sequencing used for the Y would not have been elicited in the genome sequence yet. I would speculated that repetitive regions on the X-chromosome would be a very attractive place to find genes which control ovarian reserve and maybe even genes that control spermatogenesis.
Future prospects for ovarian tissue transplantation
After ovarian transplantation, the patients were able to attempt natural conception every month without medical assistance and heterotopic sites have produced no successful pregnancies to date. Our patients preferred the chance of natural conception anyway [39,40,60,61]. In fact, the commonly held view that egg freezing is a proven technique and ovary tissue transplantation is “experimental,” is belied by the fact that all of the successful pregnancies resulting from fertility preservation in patients with cancer thus far have been from frozen ovary tissue, and none at the date of this writing have come from frozen oocytes [42]. It is generally assumed that premature ovarian failure or low ovarian reserve is related to the number of primordial follicles the woman has at birth and this number is certainly heritable and is most likely genetically determined [59]. All modern women are concerned about what is commonly referred to as their “biological clock” as they worry about the chances of conceiving by the time they have established their career and/or their marriage and their financial stability. Most of our cured patients with cancer, who have “young” ovarian tissue frozen, feel almost grateful they had cancer, because otherwise they would share this same fear all modern, liberated women have about their “biological clock.”

For patients with leukemia, or any patients in whom transplantation of prior frozen ovarian tissue might create a risk of re-introducing cancer cells, we recommend that before the cortical tissue is dissected and frozen, that all the antral follicles of the removed ovary be aspirated for germinal vesicle oocyte (GV) retrieval. These GV’s can then be partially denuded of cumulous cells, and vitrified just as for oocyte freezing (with a few minor modifications) (See Fig. 7). Allografts might be considered if ovarian tissue is available from a young woman who previously donated bone marrow to the same patient [62].
At the time of this writing, we are aware of numerous other births after implanting ovarian tissue for a total of 28 live births thus far [24,25,42,63–66]. Thus, despite initial skepticism, this technique is now gaining worldwide acceptance, and is being enthusiastically received by young women of reproductive age with cancer.
Egg and embryo vitrification
Vitrification for freezing eggs or embryos was first suggested in the mid-1980s [66,67]. However, it was not until 2005 that a highly efficient method was published, which stimulated a huge wave of justified enthusiasm for this approach to egg and embryo freezing [13,22,68,69]. The concept behind vitrification is not just its potential simplicity (like no freezing machine is required) but that could eliminate ice crystal formation completely. Instead of clinical IVF programs having to weigh carefully the risks to healthy pregnancy posed by embryo-freezing, embryos could be frozen without concern in virtually any case in which there would be a clinical advantage. With the new vitrification methodology, there is no difference in implantation potential between fresh and frozen embryos.
The vitrification protocol was designed to avoid too rapid osmotic shifts that could be caused by such high concentrations of cryoprotectant [22]. The ultra-rapid rate of cooling (−23,000 °C per minute) and the high concentration of cryoprotectant lower the freezing point dramatically and allows the ice crystallization phase to be completely avoided.
Mini-IVF with less cost and high success rates for older women
With more innovative stimulation protocols, using mild increases only in FSH level to produce a smaller number of better quality eggs, IVF not only becomes less formidable, but also yields a solution to the very obsessive question of how better IVF results can be achieved in older women. In this unusual protocol, clomiphene citrate, a competitive inhibitor of oestradiol, is used to stimulate the ovary by gently elevating FSH secretion of the pituitary gland. Continuation of clomiphene citrate beyond the usual 5 days is also able to inhibit the LH surge [70,71]. Thus, a longer stimulation can be achieved with clomiphene citrate and a premature LH surge can be prevented by the antioestrogen effect of clomiphene on the pituitary when the oestradiol begins to rise. Once adequate follicle development is noted, a gonadotrophin-releasing hormone agonist (GnRHa) is administered to trigger an LH surge for the final maturation of oocytes. This protocol is often referred to as mini-IVF [70,71].
There have been several reports of improved egg quality in IVF protocols with less medication [72–83]. Mini-IVF is less expensive and easier for the patient. With less follicles to aspirate and less eggs for the laboratory to manage, many more procedures can be performed with less effort and less cost [70,77,79,84]. The question is whether the already-demonstrated improvement in percentage of good-quality embryos with a mild stimulation protocol is sufficient to outweigh the larger number of eggs obtained with conventional stimulation [75,82]. Another more interesting question is if better results can be obtained in older women.
Is it really possible to achieve pregnancy rates with retrieval of only two or three eggs with mini-IVF that are comparable to what can be achieved with 10–20 eggs using conventional stimulation protocols, and more important can we improve results in older females?
There are many reasons for using GnRHa to trigger ovulation. It stimulates the pituitary to produce a more natural surge of LH and is potent enough to induce maturation in larger follicles. Its half-life is short and so it never stimulates small follicles. This allows the body to preserve those smaller follicles for upcoming cycles, rather than stimulate them prematurely, and allows women to cycle repeatedly without taking breaks. This is especially advantageous for older patients having limited ovarian reserve. In addition, avoiding the use of HCG also reduces the incidence of ovarian hyperstimulation syndrome (OHSS) [85–88]. All these reasons favor the use of GnRHa over the conventional use of HCG for triggering egg maturation.
The reliance on continuous clomiphene citrate, with its negative impact on the endometrium, required a simplified, cheap, reliable method for embryo cryopreservation, for which we chose the Kuwayama method [22,67]. The vitrification protocol was developed to overcome the deleterious effects of ice crystal formation that oc- curs during the slow-freezing method. With conventional slow freezing, the temperature is gradually dropped −0.3 °C per minute to create preferential ice crystal formation outside the cell. But this approach only reduces the cellular water content from 70% to 30%; there is still a fair amount of ice crystal formation intracellularly. In vitrification, the temperature drop is −23,000 °C per minute and there is no ice crystal formation whatsoever. So vitrification depends on a faster cooling rate with a higher viscosity of cryoprotectant and a miniscule cryosolution volume to get the most rapid temperatue drop and prevent ice crystal formation inside and outside the cytoplasm of the cell. Vitrification increases the possibility of using a single-embryo transfer protocol, because there is no urge from the patient to transfer more than one embryo for fear that her results with fresh embryos will be better than with cryopreserved. Furthermore, it is cheaper and easier than the conventional slow freeze methods.
In summary, maximal ovarian stimulation, which produces large numbers of eggs, results in a lower percentage of good-quality eggs (defined as implantation rate per egg) than gentle ovarian stimulation which produces fewer eggs [73,81,82]. Most importantly for older women, multiple cycles of mini-IVF with better quality embryos because of less FSH elevation give them a better result than maximal dose gonadotropins.
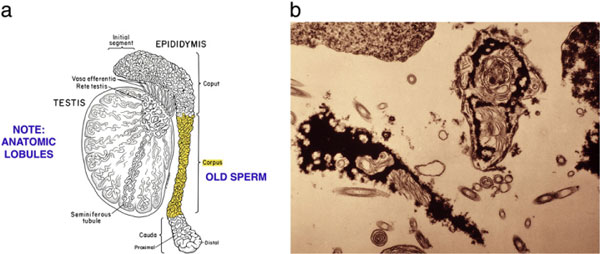
Intra-cytoplasmic sperm injection (ICSI) for azoospermic men and implications for sperm maturation
It is unknown how MESA (epididymal sperm) and TESE (testicular sperm) compare in terms of pregnancy rate in couples with azoospermia. It is also unknown whether surgically retrieved sperm has similar or worse results than ejaculated sperm either in men with low sperm count or normospermic men. Ever since ICSI was first introduced, it has been assumed that the source of the sperm does not matter, whether testis, epididymis, or ejaculate, and it has even been unclear whether the degree of spermatogenic defect has an impact on chance of pregnancy. In fact, some urologists proclaim that testis sperm is even better than ejaculated sperm and recommend TESE even when there are a few sperm in the ejaculate. This has important implications for the basic science of sperm maturation and its impact on embryo development.
We have not yet published, but have presented some interesting speculations from human results that support the concept that sperm do mature functionally when they exit the testis. We studied 374 consecutive ICSI cycles of men with obstructive azoospermia using TESE (testicular) or MESA (epididymal) sperm, and 1849 consecutive unselected cycles ICSI cycles of men without azoospermia using ejaculated sperm. The purpose of this study in which all patients underwent ICSI, was to determine the results with ICSI of ejaculated sperm versus epididymal sperm (MESA) versus testicular sperm (TESE), regardless of the spermatogenic defect.
Interestingly, we found that the MESA-ICSI cycles resulted in a significantly higher implantation rate, and significantly more clinical and ongoing pregnancies than the TESE-ICSI cycles. The results for TESE-ICSI in these men with obstructive azoospermia were similar to our results of TESE-ICSI in men with non-obstructive azoospermia. We also found no difference in results for any category of sperm count so long as ejaculated sperm were used. Furthermore, there was no difference in results between ejaculated sperm and MESA sperm. Thus, spermatogenic quantity was not an important variable, but testicular sperm seems to be inferior to both epididymal and ejaculated sperm (which are equivalent to each other). The reason that many IVF programs have poor results with epididymal sperm could be that they obtain distal sperm under the misconception that it is more mature. But in the obstructed epididymis, the distal sperm are more senescent and so the proximal most sperm surprisingly are the freshest, and will yield the best results [89].
Microsurgical TESE
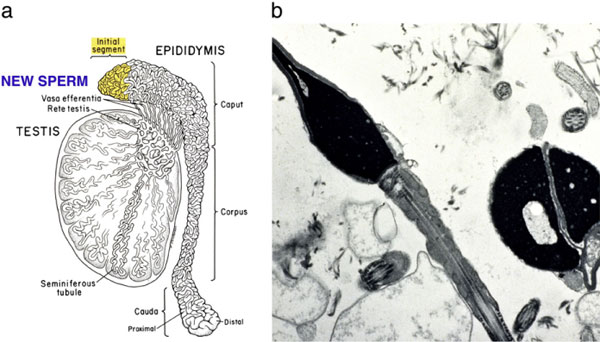
NOA is due to a severe quantitative spermatogenic defect wherein there is not enough quantity of sperm production to “spill over” into the ejaculate whereas OA is associated with normal spermatogenesis [8,89–108] (Figs. 8a and b, 9a and b). For non-obstructive azoospermia, epididymal sperm can never be retrieved, because the walls are collapsed and there is no obstruction to allow epididymal sperm collection to take place [97]. For non-obstructive azoospermia, an open testicular biopsy is performed under the microscope with the same type of local anaesthetic as for MESA, with the patient wide-awake, and with minimum postoperative discomfort. When extensive multiple biopsies from every area of the testis are performed in an effort to find sufficient sperm for TESE, a great deal of testicular damage can result, and may limit “successful” patients to only one attempt [109,110]. An attempt to limit damage by using multiple needle sticks rather than open biopsy to obtain sperm for ICSI is just as invasive and quite risky as well. Furthermore, control studies have shown that for difficult cases of non-obstructive azoospermia, where there is very limited spermatogenesis, needle biopsy is much less likely to find the rare foci of spermatogenesis than open biopsy [111,112].
The solution to cases where there are no sperm to be seen on TESE is not to look for “round spermatids” [102,113,114]. We never see round spermatids in the absence of mature spermatids, which at TESE are what just appear to be sperm [102,113,115–119]. The solution is to search for the few sperm with tails that are very sparsely and diffusely present.
Conclusions
It has been well shown over the last four decades that testis sperm undergo remarkable changes when they passage into the epididymis. Still, many andrologists have wrongfully assumed that ICSI completely avoids that need for sperm maturation. Our results indeed, do show that ICSI does not completely bypass the benefits of sperm passage into the epididymis, and it would be beneficial if sperm production could be increased enough in NOA for there to be sperm in the epididymis or ejaculate (see the last section on spermatogenic stem cells).
Sequencing the X and Y chromosome in azoospermic men to identify genetic control of spermatogenesis
In 1993, we began to study the genetic causes of male infertility via the first mapping of the Y chromosome in azoospermic males and in fertile control male populations, eventually leading to complete sequencing of the Y chromosome. This led to a better understanding of why tiny amounts of sperm are often found in the testis of azoospermic men previously thought to be making no sperm [120–127]. This ongoing genetic study of male infertility has helped to squelch the foolish but clinically popular (and wasteful) idea that hormones or varicocelectomy can improve sperm count, which is for the most part genetically determined. This specialized male chromosome, the Y chromosome, contains many genes that are involved in spermatogenesis, concentrated in a peculiar pattern of nucleotide repeats and mirror image inversions called amplicons and palin- dromes. Deletions involving these regions of the Y are found in 15% of severely infertile males and have been shown to be transmitted to male offspring via ICSI, presumably causing fertility problems in these children later in life [124,128,129]. Early mapping of these deletions were subsequently elaborated in greater detail by sequencing of the entire Y chromosome (Fig. 10).
The sequence of the Y also gives us a perspective about other male infertility genes that are widespread throughout the genome. More importantly perhaps, understanding the Y chromosome helps us to comprehend why men who are seemingly azoospermic often have some residual tiny amount of spermatogenesis that can be used for successful ICSI [97,100,130]. Many genes on the Y required for spermatogenesis have multiple copies, and even have “backup” homologues elsewhere in the genome, like DAZL and CDYL [8,131,132]. Another broader scientific benefit is that it has amplified our under- standing of sex determination in general, and has even helped to ex- plain the origin of Turner Syndrome [133]. Indeed, studies of male infertility and the Y chromosome have gone so far as to show why XO Turner females are not generally caused by the loss of an X chromosome in the female embryo, but rather by a loss of the Y chromosome in a male embryo.
Suspicion of involvement of the Y chromosome in male infertility actually arose originally from cytogenetic evidence reported over 35 years ago [134]. This karyotyping study showed grossly obvious terminal Y chromosome deletions in a very small percentage (0.5%) of azoospermic men who were otherwise phenotypically normal. During the mid-1990s, it was shown that the long arm of the Y chromosome indeed does contain not one but many distinct deletion intervals and at least 60 genes belonging to nine gene families whose exclusive function is in spermatogenesis [120–123,125–127,135]. The Y deletions have been divided clinically into three groups, but this classification is actually naïve in most instances.
AZFa
![Figure 10: Early map of major Y chromosome deletions in azoospermic men and the diversity of pathologic defects [118].](https://www.infertile.com/wp-content/uploads/2014/11/bba-12.jpg)
The AZFa region differs from the AZFb and AZFc regions because of its non-repetitive structure and its low deletion frequency (Fig. 11). AZFa deletions are very uncommon and only a few rare patients have been described [135–140]. However, studies of this region are highly useful in understanding the genetic basis of male infertility. The AZFa region spans ~800 kilobases (kb) and contains two functional single-copy genes: USP9Y and DBY [140,141]. A point mutation in USP9Y represented the first case of a point mutation rather than a large micro-deletion on the Y chromosome associated with spermatogenic failure [140]. The lack of sequence repeats which plague the rest of the Y chromosome, made this particular region of the Y amenable to such a point mutation search.
The AZFa region provides a good model for the interaction and overlapping functions of multiple genes, which sheds light on the polygenic nature of the genetic control of spermatogenesis. When the entire AZFa region is deleted, taking out both DBY and USP9Y, the spermatogenic defect is severe and the patient is always azoospermic. In contrast, when only one gene is affected, such as in the patient with loss of USP9Y function due to a specific point mutation, this results in a less severe phenotype of maturation arrest with a few pachytene spermatocytes developing into a few mature sperm in some seminiferous tubules.
AZFb
![Figure 11: Simplified diagram of the main areas for Y chromosome microdeletions, including some that are less frequently studied, such as b2–b3 [140].](https://www.infertile.com/wp-content/uploads/2014/11/bba-13.jpg)
Deletions of the AZFb region are slightly more common than deletions of the AZFa region but are still found in a very small percent of azoospermic men [135,142–145]. Interestingly, all men with dele- tions of AZFb described to date are azoospermic and also show complete absence of spermatozoa in the testis [131,135,143–150]. Therefore, similar to AZFa deletions, there are no reports on transmis- sion of an AZFb deletion to ICSI offspring. Many functionally active genes are clustered within the AZFb region [147,151,152]. Because of the presence of multiple sequence repeats within this region, in contrast to AZFa, efforts to define the AZFb region precisely had been hampered until more recently [127,143].
In fact, the region frequently referred to simplistically as AZFb overlaps AZFc and has a massive number of repeat copies of genes and pseudogenes (RBMY, PRY, TTTY) arranged in a complex of palindromes (inverted repeat sequences). That is why we technically object to this commonly used terminology of “AZFb” and prefer to refer to the P5/P1 deletion.
AZFc
The most commonly deleted and best-studied region on the Y chromosome is the AZFc region. Deletion of the AZFc region is found in approximately 12% of azoospermic men and in 6% of severely oligozoospermic men [8,120–122,153]. This region is constructed from massive areas of absolute sequence identity called amplicons which are arranged in direct repeats, and inverted repeats, or palindromes. The AZFc region spans 3.5 Mb (huge but not quite as huge as AZFb with its massive 8 Mb length), and contains seven separate families of genes with a total of 19 genes that are all exclusively expressed in the testis. Once called a “microdeletion,” AZFc, is actually a huge section of DNA, but just not huge enough to show up with karyotyping. Interestingly, absence of this large 3.5-Mb AZFc chunk of the Y chromosome, as well as the much larger even AZFb region, seems to have no other deleterious effect on the male except upon spermatogenesis, exemplifying the remarkably specialized function of this region of the Y chromosome [121]. These genes only affect spermatogenesis and nothing else.
The DAZ gene family, which is one of the seven gene families located within AZFc, was one of the first spermatogenesis genes identified on the human Y chromosome and comes in four almost identical copies [120,125]. The human DAZ genes were shown to be transcribed specifically in spermatogonia and in early primary spermatocytes [154]. Interestingly, autosomal homologues of DAZ in other species were also shown to be involved in control of spermatogenesis, supporting an essential role of this gene in male fertility in humans as well as almost all other animals. Autosomal homologues of DAZ have been found in Drosophila (termed Boule), in mice (termed Dazl), in frogs (termed Xdazl) and even in worms (termed daz-1) [155–160]. Therefore, DAZ is the most ancient and well conserved spermatogenesis gene. In contrast to humans, the DAZ gene in these other species is single copy and located on an autosome rather than on the Y chromosome.
The human also retains an autosomal homologue of DAZ called DAZL, which is located on chromosome 3 [125]. During evolution, sometime after the split of Old and New World monkeys approximately 30 million years ago, the DAZL gene was transposed to the Y chromosome like a simple translocation event. Once autosomal DAZL was transposed from chromosome 3 to the Y, it was amplified into four copies. The exact interaction and possible functional overlap between these members of this interesting gene family perhaps holds the clue to the peculiar finding of a few surviving sperm in the testes of azoospermic men because of genetic “backup.”
The Y chromosome, with its lack of recombination during meiosis, represents a safe harbor for the proliferation of genes that are beneficial to the male but detrimental to the female. This is how the Y chromosome, over evolutionary time, tends to accumulate genes from throughout the entire rest of the genome that control spermatogenesis. A degradation of its X homologues due to failure of recombination occurs at the same time.
Deletions of the entire AZFc region result in loss of all four DAZ copies, but most of these men still have some minute amount of spermatogenesis, speculatively because of backup from DAZL on chromosome 3. Men with a deletion of only two DAZ genes are less affected than men with a deletion of all four copies [161–165]. Disruption of different genes or disruption of some genes of a gene family can result in different degrees of spermatogenic failure.
Mechanism of de-novo Y chromosome deletions
Deletions of the Y are caused by “illegitimate” homologous recombination between highly similar or identical sequences, which are found on the Y in great abundance. For example, homologous recom- bination between two identical sequence stretches results in dropout of the intervening AZFa region [141,166,167]. Since these sequences of identical nucleotide repeats are shorter than those in AZFc or AZFb, AZFa deletions are very rare. For AZFc the substrates for homologous recombination are two repeats that are over 99.9% identical and as long as 229 kb in length [121]. Therefore, deletions of AZFc, caused by homologous recombination between 229 kb repeats, are far more common than deletions of AZFa which are caused by repeats of only 10 kb in length. The very repetitive nature of the Y chromosome seems to be the cause of its instability over an evolutionary time frame, but it is also the method that the Y has adopted (albeit inefficient) for its survival, called “gene conversion” [168].
What is equally fascinating is how we survived at all, as our X homologous genes on the Y chromosome deteriorated because of failure of meiosis with its non-recombining mate [169,170]. In fact, this deterioration of X homologous Y genes was the whole reason for the evolution of “X inactivation,” to put males and females on an equal footing despite different gene dosages. This gives us a better understanding of the evolution in time over and over again of sex chromosomes.
Evolution and genetic constitution of the human Y chromosome
Over the past 240–320 million years of mammalian evolution, the X and Y chromosomes have evolved from what was originally a pair of ordinary autosomes about the size of chromosome 7 or 8. During that evolution, just as most of the ancestral X genes were decaying on the Y because of the lack of meiotic recombination, at the same time genes which control spermatogenesis arrived on the Y from autosomes. Once on the Y, these formerly autosomal genes amplified into multiple copies, and inversions through the process called “gene conversion” [125,168,171]. Other spermatogenesis genes on the Y actually originated there, such as RBMY, and have persisted in their original position as on the X [172–175]. The ancestral gene that remained on the X chromosome (RBMX) retained its widespread cellular functions, whereas RBMY, which persisted on the receding Y chromosome, evolved a male-specific function in spermatogenesis again because of the advantage of having male specific genes on a chromosome found only in males [174,176–178].
How does the male specific Y survive at all? The answer is “gene conversion” [168]. When autosomes recombine during normal meiosis, DNA is exchanged in a way that the accumulated mutational errors of life get corrected in the germ cells via this DNA exchange. In a sense, the autosomes have “sex” with each other. This correctional meiosis cannot occur with the Y chromosome. Instead, the Y has “sex” with itself. That is, during meiosis, the like sequence repeats of the Y just “recombine” in a sense with each other, and that is the function of the palindromes.
The Y chromosome and spermatogenesis in humans and in apes
Most intriguing is to compare the human Y chromosome to the chimpanzee Y chromosome, both of which have been fully and accurately sequenced [127,179–181]. Some interesting differences are noted between the human Y and the chimpanzee Y [181]. Firstly, the chimpanzee Y has many more amplicons and palindromes than the human, but nonetheless it has much fewer ampliconic genes (25 compared to 60). So the increased sperm production of chimpanzees cannot simply be explained by the presence of more testis specific genes on their Y chromosome. But interestingly, the chimpanzee Y is missing a gene (PRY) that is present on AZFc in two copies in the human. Speculatively, the PRY gene, which humans have and chimpanzees do not have, could be a suppressor of spermatogenesis. It is fascinating that this gene has also been found to be present in only one copy in rare humans who have incredibly high sperm counts, approaching half a billion [182].
Other male infertility genes not on the Y chromosome
Although most research into the genetics of male infertility has focused on the Y chromosome. Its counterpart, the X chromosome, also seems to be an ideal locus for spermatogenesis genes. Like the Y chromosome, the X chromosome is present as a single copy in the heterogametic XY male. Therefore, any mutation favorable to spermatogenesis is more likely to spread and be expressed phenotypically in future generations of males if it is on the X.
Of course, in the egg, i.e., in the female, the X chromosome undergoes normal meiosis and repair with its paired X mate, unlike the Y. However, the X spends one-third of its evolutionary life in the testis, and the X does not get to undergo normal meiotic repair in the testis. So it cannot escape completely some of the problems that the Y solves by constructing amplicons and palindromes. I would suspect that ampliconic regions on the X-chromosome, which have not yet been well characterized, could have a rich complement of spermatogenesis genes. It seems very likely that evolution has conferred on the human X chromosome also a large portion of the burden for spermatogenesis [183].
What does the future hold?
It is commonly thought by laymen and others that the human genome has been completely sequenced. Of course this is not true. Routine shotgun sequencing is ineffective for elucidating ampliconic and palindromic regions of multiple sequence repeats, such as dominate the Y chromosome. It is certain that there are many ampliconic sequences throughout the genome that have not been elucidated. Also, it is possible that with faster and cheaper sequencing of the entire genome, the exome of the autosomes will reveal the rest of the genes which control spermatogenesis.
Spermatogenic stem cell culture and transplantation to salvage spermatogenesis in pre-pubertal boys about to undergo sterilizing chemotherapy
It was first shown by Ralph Brinster that spermatogenic stem cells derived from the tissue of normal mice can be transferred via the rete testis into mice with no spermatogenesis, and they will gradually populate stem cell niches all along the seminiferous tubules of large areas of the previously sterile recipient mouse testis and result in complete spermatogenesis and ultimately in normal offspring [184,185]. Subsequently spermatogenic stem cells from a variety of species were successfully transplanted into SCID mice. The closer the phylum of the donor spermatogenic stem cells to the sterilized recipient mouse, the farther along the spermatogenesis of the transplanted germ cells would develop. For example, rat stem cells would develop mature sperm in the SCID mouse but at the rate of rat spermatogenesis rather than mouse spermatogenesis. For more distant phyla, the final stages of spermatogenesis are not seen although earlier stages are well supported [186–190]. Frozen spermatogenic stem cells did just as well as fresh [191]. In this ground breaking basic science work, Brinster’s lab set the stage for a potential clinical application for preserving fertility in cancer patients, particularly in pre-pubertal boys, and also set the stage for a method finally of the raising the sperm count of azoospermic or severely oligospermic men [192,193].
Over the last three decades, effective cancer treatments have improved the survival rates for many types of cancer. In children, the survival rate for all cancers combined improved from 58% to 80% [194]. Currently it is estimated that 1 in 250 young adults between 20 and 29 years of age is a long-term survivor of childhood cancer [195,196]. The adverse side effects of their cancer treatment include gonadal failure and sterility, and great efforts are now being made to find a way to prevent or modify these complications of cancer therapy [197]. For post-pubertal boys or men, it is simple just to cryopreserve mature sperm found in ejaculate [198]. For girls or women ovarian cortical strips can be cryopreserved [199]. But for prepubescent boys, the only way to preserve their future fertility is through testis biopsy, spermatogonial stem cell culture and amplification, cryopreservation of spermatogonial stem cells, and their subsequent transfer back to the patient when he is an adult [200–204].
Recent studies have shown that long-term culture of spermatogenic stem cells prior to transplanting via efferent ducts or rete testis to a sterile recipient allows their amplification in number so as to get a better ultimate result [192,202,205,206]. Since these stem cells represent .03% of germ cells and 1.25% of spermatogonia, in vitro culture and amplification of cryopreserved spermatogenic stem cells will allow this clinical application of preserving fertility for pre-pubertal male cancer patients, and possibly some day of converting very oligospermic or azoospermic males to normospermia.
Conclusion
In summary, what are the new science-based treatments on the horizon for fertility? New technology in cryopreservation via vitrification allows us to remove ovary tissue and freeze it to protect it from sterilizing cancer treatment in young women, as well as to freeze individual mature eggs. It also allows us to stop the aging of the ovary and eggs, which is the single major cause of the current worldwide infertility epidemic. For the nagging problem of the older woman, a milder increase in FSH allows better quality eggs to be retrieved for women in their 40s at a more affordable cost and less effort. Thus multiple cycles can be performed for retrieval of only the few best eggs, accumulated and stored up over a year by vitrification, and eventually achieve pregnancy rates similar to that of young women. Although ICSI would seem to be already a very mature and established treatment, the role of sperm quality in determining ICSI results has been obscure. By establishing that epididymal sperm yields better results than testis sperm, we are just beginning to tap into this question. Results of ICSI for azoospermic men show that testis sperm are indeed inferior to epididymal sperm regardless of quality or quantity of spermatogenesis. Sequencing of the X and Y chromosomes reveal that spermatogenic genes are concentrated in remarkable ampliconic repeat stretches of DNA in the Y chromosome and we predict a similar concentration of spermatogenic genes on the X chromosome and throughout the rest of the genome. This new molecular information explains why conventional treatments to raise sperm count fail, and also why 60% of even azoospermic men have minute amounts of sperm. Finally, spermatogenic stem cell culture and transplantation in humans can preserve the fertility of prepubertal boys about to undergo cancer treatment, and also by means of stem cell amplification can increase sperm count even in severely oligospermic or azoospermic men.
Addendum
There are other areas of future promise for fertility treatment that are either more speculative, or are beyond this author’s area of expertise. I personally find to be useless the very numerous “reviews” of literature, even with statistically valid meta-analysis, which are outside of the personal experience and true expertise of the author. Therefore, I have not included such review in this closing chapter except for this addendum. I will briefly mention them here, along with opinions about their potential future value, however useless those opinions might be.
The potential clinical treatment application of sequencing the X and Y chromosomes in infertile men is often questioned. Will it lead to finding a genetic cause in all men? Will it lead to genetic or other treatments for male infertility like germ line therapy, or new drugs? That question, often asked, is like asking Einstein whether his 1905 paper would eventually lead to television, or whether silicone research in the 40s would lead to iPhones and iPads. The future practical value of understanding a phenomenon is often very clouded and such speculation can be foolish. But I can be absolutely 100% certain that there will eventually be a practical use in male infertility management for our sequencing of the X and the Y chromosomes and indeed re-sequencing the whole genome to get accurate information about the heretofore hidden blank spots.
Pre-implantation genetic diagnosis (PGD) or screening (PGS) is being promoted more and more furiously. No one doubts its value for single gene disorders. But its value for chromosomal screening of embryos for chromosomal errors has been disappointing. Randomized prospective control studies have shown day 3 biopsy with FISH evaluation of blastomeres to be useless, and indeed harmful. Lately, however, with the new technology of trophectoderm laser assisted blastocyst biopsy and molecular karyotyping via whole genome amplification and either comparative genomic hybridization (CGH) or single nucleotide polymorphism (SNP) arrays, the concept of PGS is now being re-energized. But the true practical value of this fascinating field may be dismissed with a simple “thought experiment”: you simply cannot improve pregnancy rate with selection. You can only decrease the pregnancy rate by either an error in diagnosis or by damage to the biopsied embryo. But you cannot improve the ultimate pregnancy rate over simply vitrifying and eventually transferring all the embryos.
The biggest practical question for futuristic treatments is whether women will take advantage of our ability to preserve their fertility with egg or ovary freezing at a young age, in their early or mid-twenties. The technology is proven and available for ending the infertility epidemic. But we typically see only 35–40 year old women, often who have just broken up with their boyfriend, come to clinic to request fertility preservation, when in truth in the modern era, every woman over 21 years of age should be given this opportunity if she so chooses. But none of them do.
References
- M.P. Connolly, M.S. Pollard, S. Hoorens, B.R. Kaplan, S.P. Oskowitz, S.J. Silber, Long-term economic benefits attributed to IVF-conceived children: a lifetime tax calculation, Am. J. Manag. Care 14 (9) (2008) 598–604.
- S.J. Silber, How to Get Pregnant: The Classic Guide to Overcoming Infertility, Completely Revised and Updated, second ed. Little, Brown, Boston, MA, 2007.
- SART, Assisted Reproductive Technology Success Rates. National Summary and Fertility Clinic Reports, Center for Disease Control and Prevention, Atlanta, GA, 2005.
- S.J. Silber, The relationship of abnormal semen parameters to male fertility, Hum. Reprod. 4 (8) (1989) 947–953.
- S.J. Silber, Evaluation and treatment of male infertility, Clin. Obstet. Gynecol. 43 (4) (2000) 854–888.
- S.J. Silber, The varicocele dilemma, Hum. Reprod. Update 7 (1) (2001) 70–77.
- J.W.vanderSteeg,P.Steures,M.J.C.Eijkemans,J.D.F.Habbema,P.G.Hompes,J.A. Kremer, L. van der Leeuw-Harmsen, P.M. Bossuyt, S. Repping, S.J. Silber, B.W. Mol, F. van der Veen, Collaborative Effort for Clinical Evaluation in Reproductive Medicine Study Group, Role of semen analysis in subfertile couples, Fertil. Steril. 95 (3) (2011) 1013–1019.
- S.J. Silber, The Y chromosome in the era of intracytoplasmic sperm injection: a personal review, Fertil. Steril. 95 (8) (2011) 2439–2448.
- S.J. Silber, Microsurgical aspects of varicocele, Fertil. Steril. 31 (2) (1979)230–232.
- S.J. Silber, H.E. Grotjan, Microscopic vasectomy reversal 30 years later: a summary of 4010 cases by the same surgeon, J. Androl. 25 (6) (2004) 845–859.
- S.J. Silber, Testis biopsy and the infertile male, in: P.E. Patton, D.E. Battaglia (Eds.), Contemporary Endocrinology: Office Andrology, Humana Press Inc., New Jersey, 2005, pp. 215–240.
- A.R. Baerwald, G.P. Adams, R.A. Pierson, Ovarian antral folliculogenesis during the human menstrual cycle: a review, Hum. Reprod. Update 18 (1) (2012) 73–91.
- R. Homburg, F. van der Veen, S.J. Silber, Oocyte vitrification—women’s emancipation set in stone, Fertil. Steril. 91 (2009) 1319–1320.
- S.J. Silber, N. Kagawa, M. Kuwayama, R. Gosden, Duration of fertility after fresh and frozen ovary transplantation, Fertil. Steril. 94 (6) (2010) 2191–2196.
- S.J. Silber, Ovary Cryopreservation and Transplantation, Mol. Hum. Reprod. http://dx.doi.org/10.1016/j.bbadis.2012.10.004.
- W.D. Mosher, Fecundity and infertility in the United States 1965–1982, Adv. Data 1 (1985) 1.
- S. Geber, T. Paraschos, G. Atkinson, R. Margara, R.M.L. Winston, Results of IVF in patients with endometriosis: the severity of the disease does not affect the outcome, or the incidence of miscarriage, Hum. Reprod. 10 (6) (1995) 1507–1511.
- S.J. Silber, Z. Nagy, P. Devroey, M. Camus, A.C. van Steirteghem, The effect of female age and ovarian reserve on pregnancy rate in male infertility: treatment of azoospermia with sperm retrieval and intracytoplasmic sperm injection, Hum. Reprod. 12 (12) (1997) 2693–2700.
- C.P. Biacchiardi, L.D. Paine, M. Camanni, F. Deltetto, E.M. Delpiano, G.L. Marchino, G. Gennarelli, A. Revelli, Laparoscopic stripping of endometriomas negatively affects ovarian follicular reserve even if performed by experienced surgeons, Reprod. Biomed. Online 23 (6) (2011) 740–746.
- E. Moll, F. van der Veen, M. van Wely, The role of metformin in polycystic ovary syndrome: a systematic review, Hum. Reprod. Update 13 (6) (2007) 527–537.
- R.G. Gosden, D.T. Baird, J.C. Wade, R. Webb, Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C, Hum. Reprod. 9 (4) (1994) 597–603.
- M. Kuwayama, G. Vajta, O. Kato, S.P. Leibo, Highly efficient vitrification method for cryopreservation of human oocytes, Reprod. Biomed. Online 11 (3) (2005) 300–308.
- S.J. Silber, K.M. Lenahan, D.J. Levine, J.A. Pineda, K.S. Gorman, M.J. Friez, E.C. Crawford, R.G. Gosden, Ovarian transplantation between monozygotic twins discordant for premature ovarian failure, N. Eng. J. Med. 353 (1) (2005) 58–63.
- J. Donnez, M.M. Dolmans, D. Demylle, P. Jadoul, C. Pirard, J. Squifflet, B. Martinez-Madrid, A. van Langendonckt, Livebirth after orthotopic transplantation of cryopreserved ovarian tissue, Lancet 364 (9443) (2004) 1405–1410.
- D. Meirow, J. Levron, T. Eldar-Geva, I. Hardan, E. Fridman, Y. Zalel, E. Schiff, J. Dor, Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy, N. Engl. J. Med. 353 (3) (2005) 318–321.
- S.J. Silber, R.G. Gosden, Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure, N. Eng. J. Med. 356 (2007) 1382–1384.
- S.J. Silber, M. DeRosa, J. Pineda, K. Lenahan, D. Grenia, K. Gorman, R.G. Gosden, A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation, Hum. Reprod. 23 (7) (2008) 1531–1537.
- S.J. Silber, G. Grudzinskas, R.G. Gosden, Successful pregnancy after microsurgical transplantation of an intact ovary, N. Eng. J. Med. 359 (24) (2008) 2617–2618.
- W.H. Wallace, H.O. Critchley, R.A. Anderson, Optimizing reproductive outcome in children and young people with cancer, J. Clin. Oncol. 30 (1) (2012) 3–5.
- M.A. Bedaiwy, T. Falcone, Harvesting and autotransplantation of vascularized ovarian grafts: approaches and techniques, Reprod. Biomed. Online 14 (3) (2007) 360–371.
- S.J. Silber, Transplantation of a human testis for anorchia, Fertil. Steril. 30 (2) (1978) 181–187.
- R.G. Gosden, E. Telfer, M.J. Faddy, D.J. Brook, Ovarian cyclicity and follicular recruitment in unilaterally ovariectomized mice, J. Reprod. Fertil. 87 (1) (1989) 257–264.
- M.J. Faddy, R.G. Gosden, A. Gougeon, S.J. Richardson, J.F. Nelson, Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause, Hum. Reprod. 7 (10) (1992) 1342–1346.
- D.A. Gook, D.H. Edgar, C. Stern, Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1,2-propanediol, Hum. Reprod. 14 (8) (1999) 2061–2068.
- H. Newton, Y. Aubard, A. Rutherford, V. Sharma, R. Gosden, Low temperature storage and grafting of human ovarian tissue, Hum. Reprod. 11 (7) (1996) 1487–1491.
- N. Kagawa, S. Silber, M. Kuwayama, Successful vitrification of bovine and human ovarian tissue, Reprod. Biomed. Online 18 (4) (2009) 568–577.
- V. Keros, S. Xella, K. Hultenby, K. Pettersson, M. Sheikhi, A. Volpe, J. Hreinsson, O. Hovatta, Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue, Hum. Reprod. 24 (7) (2009) 1670–1683.
- F. Fuentes, R. Dubettier, Air separation method and plant, United States Patent. (2004) Patent No. US 6,776,005 B2.
- K. Oktay, E. Buyuk, L. Veeck, N. Zaninovic, K. Xu, T. Takeuchi, M. Opsahl, Z.
- Rosenwaks, Embryo development after heterotopic transplantation of cryopreserved ovarian tissue, Lancet 363 (9412) (2004) 837–840.
- M. Rosendahl, A. Loft, A.G. Byskov, S. Ziebe, K.T.L. Schmidt, A.N. Andersen, C. Ottosen, C.Y. Andersen, Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report, Hum. Reprod. 21 (8) (2006) 2006–2009.
- D.T. Baird, R. Webb, B.K. Campbell, L.M. Harkness, R.G. Gosden, Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 °C, Endocrinology 140 (1) (1999) 462–471.
- J. Donnez, S. Silber, C.Y. Andersen, I. Demeestere, P. Piver, D. Meirow, A. Pellicer, M.M. Dolmans, Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births, Ann. Med. 43 (6) (2011) 437–450.
- W.A. Bleyer, The impact of childhood cancer on the United State and the world, CA Cancer J. Clin. 40 (6) (1990) 355–367.
- [In: L.A.G. Ries, M.A. Smith, J.G. Gurney, M. Linet, T. Tamra, J.L. Young, G.R. Bunin (Eds.), Cancer incidence and survival among children and adolescents: United States SEER program, 1976–1995, National Cancer Institue, 1999.
- J.S. Jeruss, T.K. Woodruff, Preservation of fertility in patients with cancer, N. Engl. J. Med. 360 (9) (2009) 902–911.
- R.A. Anderson, A.P. Themmen, A. Al-Qahtani, N.P. Groome, D.A. Cameron, The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer, Hum. Reprod. 21 (10) (2006) 2583–2592.
- R.A. Anderson, D.A. Cameron, Assessment of the effect of chemotherapy on ovarian function in women with breast cancer, J. Clin. Oncol. 25 (12) (2007) 1630–1631 (Author reply 1632).
- E.C. Larsen, J. Muller, K. Schmiegelow, C. Rechnitzer, A.N. Andersen, Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer, J. Clin. Endocrinol. Metab. 88 (11) (2003) 5307–5314.
- S.J. Lee, L.R. Schover, A.H. Partridge, P. Patrizio, W.H. Wallace, K. Hagerty, L.N. Beck, L.V. Brennan, K. Oktay, American Society of Clinical Oncology, American Society of Clinical Oncology recommendations on fertility preservation in cancer patients, J. Clin. Oncol. 24 (18) (2006) 2917–2931.
- C.Y. Andersen, M. Rosendahl, A.G. Byskov, A. Loft, C. Ottosen, M. Dueholm, K.L.T. Schmidt, A.N. Andersen, E. Ernst, Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue, Hum. Reprod. 23 (10) (2008) 2266–2272.
- C.B. Coulam, S.C. Adamson, J.F. Annegers, Incidence of premature ovarian failure, Obstet. Gynecol. 67 (4) (1986) 604–606.
- E. Riboli, K.J. Hunt, N. Slimani, P. Ferrari, T. Norat, M. Fahey, U.R. Charrondiere, B. Hemon, C. Casagrande, J. Vignat, K. Overvad, A. Tjonneland, F. Clavel-Chapelon, A. Thiebaut, J. Wahrendorf, H. Boeing, D. Trichopoulos, A. Trichopoulou, P. Vineis, D. Palli, H.B. Bueno-De-Mesquita, P.H. Peeters, E. Lund, D. Engeset, C.A. Gonzalez, A. Barricarte, G. Berglund, G. Hallmans, N.E. Day, T.J. Key, R. Kaaks, R. Saracci, European Prospective Investigation into Cancer and Nutrition (EPIC): study of populations and data collection, Public Health Nutr. 5 (6B) (2002) 1113–1124.
- J.L. Luborsky, P. Meyer, M.F. Sowers, E.B. Gold, N. Santoro, Premature menopause in a multi-ethnic population study of the menopause transition, Hum. Reprod. 18 (1) (2003) 199–206.
- D. Goswami, G.S. Conway, Premature ovarian failure, Hum. Reprod. Update 11 (4) (2005) 391–410.
- H. Snieder, A.J. MacGregor, T.D. Spector, Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause, J. Clin. Endocrinol. Metab. 83 (6) (1998) 1875–1880.
- J.P. de Bruin, H. Bovenhuis, P.A. van Noord, P.L. Pearson, J.A. van Arendonk, E.R. te Velde, W.W. Kuurman, M. Dorland, The role of genetic factors in age at natural menopause, Hum. Reprod. 16 (9) (2001) 2014–2018.
- K.M. van Asselt, H.S. Kok, P.L. Pearson, J.S. Dubas, P.H. Peeters, E.R. Te Velde, P.A. van Noord, Heritability of menopausal age in mothers and daughters, Fertil. Steril. 82 (5) (2004) 1348–1351.
- L.L. Su, Monoamniotic twins: diagnosis and management, Acta Obstet. Gynecol. Scand. 81 (11) (2002) 995–1000.
- R.G. Gosden, S.A. Treloar, N.G. Martin, L.F. Cherkas, T.D. Spector, M.J. Faddy, S.J. Silber, Prevalence of premature ovarian failure in monozygotic and dizygotic twins, Hum. Reprod. 22 (2) (2007) 610–615.
- C.G. Hilders, A.G. Baranski, L. Peters, A. Ramkhelawan, J.B. Trimbos, Successful human ovarian autotransplantation to the upper arm, Cancer 101 (12) (2004) 2771–2778.
- S.S. Kim, I.T. Hwang, H.C. Lee, Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function, Fertil. Steril. 82 (4) (2004) 930–932.
- V.T. Armenti, J.S. Radomski, M.J. Moritz, L.Z. Philips, C.H. McGrory, L.A. Coscia, Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation, Clin. Transplant. (2000) 123–134.
- I. Demeestere, P. Simon, S. Emiliani, A. Delbaere, Y. Englert, Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease, Oncologist 12 (12) (2007) 1437–1442.
- M. Sanchez-Serrano, J. Crespo, V. Mirabet, A.C. Cobo, M.J. Escriba, C. Simon, A. Pellicer, Twins born after transplantation of ovarian cortical tissue and oocyte vitrification, Fertil. Steril. 93 (1) (2010) 268.e11–268.e13.
- P. Piver, C. Amiot, G. Agnani, J.C. Pech, P.S. Rohrilich, E. Vidal, Y. Aubard, C. Roux, Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue, Hum. Reprod. 24 (15) (2009) 10–35.
- W.F. Rall, Factors affecting the survival of mouse embryos cryopreserved by vitrification, Cryobiology 24 (5) (1987) 387–402.
- G.M. Fahy, D.R. MacFarlane, C.A. Angell, H.T. Meryman, Vitrification as an approach to cryopreservation, Cryobiology 21 (4) (1984) 407–426.
- M. Kuwayama, Highly efficient vitrification for cryopreservation of human oo- cytes and embryos: the Cryotop method, Theriogenology 67 (1) (2007) 73–80.
- K.P. Katayama, J. Stehlik, M. Kuwayama, O. Kato, E. Stehlik, High survival rate of vitrified human oocytes results in clinical pregnancy, Fertil. Steril. 80 (1) (2003) 223–224.
- S. Teramoto, O. Kato, Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study, Reprod. Biomed. Online 15 (2) (2007) 134–138.
- J. Zhang, L. Chang, Y. Sone, S. Silber, Minimal ovarian stimulation (mini-IVF) for IVF utilizing vitrification and cryopreserved embryo transfer, Reprod. Biomed. Online 21 (4) (2010) 485–495.
- E.B. Baart, E. Martini, M.J. Eijkemans, D. Van Opstal, N.G.M. Beckers, A. Verhoeff, N.S. Macklon, B.C.J.M. Fauser, Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in human preimplantation embryo: a randomized controlled trial, Hum. Reprod. 22 (4) (2007) 980–988.
- J. Collins, Mild stimulation for in vitro fertilization: making progress downward, Hum. Reprod. Update 15 (1) (2009) 1–3.
- D. de Jong, N.S. Macklon, B.C. Fauser, A pilot study involving minimal ovarian stimulation for in vitro fertilization: extending the ‘follicle-stimulating hormone window’ combined with the gonadotropin-releasing hormone antagonist cetrorelix, Fertil. Steril. 73 (5) (2000) 1051–1054.
- F. Devreker, E. Pogonici, V. De Maertelaer, P. Revelard, M. Van den Bergh, Y. Englert, Selection of good embryos for transfer depends on embryo cohort size: implications for the ‘mild ovarian stimulation’ debate, Hum. Reprod. 14 (12) (1999) 3002–3008.
- B.C. Fauser, P. Devroey, S.S. Yen, R. Gosden, W.F. Crowley Jr., D.T. Baird, P. Bouchard, Minimal ovarian stimulation for IVF: appraisal of potential benefits and drawbacks, Hum. Reprod. 14 (11) (1999) 2681–2686.
- E.M. Heijnen, M.J. Eijkemans, C. De Klerk, S. Polinder, N.G. Beckers, E.R. Klinkert, F.J. Broekmans, J. Passchier, E.R. Te Velde, N.S. Macklon, B.C. Fauser, A mild treatment strategy for in-vitro fertilisation: a randomized non-inferiority trial, Lancet 369 (9563) (2007) 743–749.
- M.J. Pelinck, N.E.A. Vogel, E.G.J.M. Arts, A.H.M. Simons, M.J. Heineman, A. Hoek, Cumulative pregnancy rates after maximum of nine cycles of modified natural cycle IVF and analysis of patient drop-out: a cohort study, Hum. Reprod. 22 (9) (2007) 2463–2470.
- S. Polinder, E.M.E.W. Heijnen, N.S. Mackon, J.D.F. Habbema, B.J.C.M. Fauser, M.J.C. Eijkemans, Cost-effectiveness of a mild compared with a standard strategy for IVF: a randomized comparison using cumulative term live birth as the primary endpoint, Hum. Reprod. 23 (2) (2008) 316–323.
- M.H. van der Gaast, M.J. Eijkemans, J.B. van der Net, E.J. de Boer, C.W. Burger, F.E. van Leeuwen, B.C. Fauser, N.S. Macklon, Optimum number of oocytes for a successful first IVF treatment cycle, Reprod. Biomed. Online 13 (4) (2006) 476–480.
- M.F.G. Verberg, M.J.C. Eijkemans, N.S. Macklon, E.M.E.W. Heijen, E.B. Baart, F.P. Hohmann, B.C.J.M. Fauser, F.J. Broekmans, The clinical significance of the retrieval of a low number of oocytes following mild ovarian stimulation for IVF: a meta-analysis, Hum. Reprod. Update 12 (1) (2009) 5–12.
- M.F.G. Verberg, N.S. Macklon, G. Nargund, R. Frydman, P. Devroey, F.J. Broekmans, B.C.J.M. Fauser, Mild ovarian stimulation for IVF, Hum. Reprod. Update 15 (1) (2009) 13–29.
- M.F. Verberg, M.J. Eijkemans, E.M. Heijnen, F.J. Broekmans, C. de Klerk, B.C. Fauser, N.S. Macklon, Why do couples drop-out from IVF treatment? A prospective cohort study, Hum. Reprod. 23 (9) (2008) 2050–2055.
- J.H. Check, Mild ovarian stimulation, J. Assist. Reprod. Genet. 24 (12) (2007) 621–627.
- J.C. Emperaire, A. Ruffie, A.J. Audebert, Ovulation induction by endogenous LH re- leased by the administration of an LHRH agonist after follicular stimulation for in vitro fertilization, J. Gynecol. Obstet. Biol. Reprod. (Paris) 21 (5) (1992) 489–494.
- J. Gerris, A. De Vits, M. Joostens, E. Van Royen, Triggering of ovulation in human menopausal gonadotrophin-stimulated cycles: comparison between intrave- nously administered gonadotrophin-releasing hormone (100 and 500 μg), GnRH agonist (buserelin, 500 μg) and human chorionic gonadotrophin (10,000), Hum. Reprod. 10 (1) (1995) 56–62.
- G. Griesinger, K. Diedrich, P. Devroey, E.M. Kolibianakis, GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis, Hum. Reprod. Update 12 (2) (2006) 159–168.
- P. Humaidan, H. Ejdrup Bredkjar, L. Bungum, M. Bungum, M.L. Grondahl, L. Westergaard, C. Yding Andersen, GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study, Hum. Reprod. 20 (5) (2005) 1213–1220.
- Tournaye, P. Devroey, J. Liu, Z. Nagy, W. Lissens, A. van Steirteghem, Microsurgical epididymal sperm aspiration and intracytoplasmic sperm injection: a new effective approach to infertility as a result of congenital bilateral absence of the vas deferens, Fertil. Steril. 61 (6) (1994) 1045–1051.
- G. Palermo, H. Joris, P. Devroey, A. Van Steirteghem, Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte, Lancet 340 (8810) (1992) 17–18.
- P. Devroey, S. Silber, Z. Nagy, J. Liu, H. Tournaye, H. Joris, G. Verheyen, A. van Steirteghem, Ongoing pregnancies and birth after intracytoplasmic sperm injection with frozen–thawed epididymal spermatozoa, Hum. Reprod. 10 (4) (1995) 903–906.
- P. Devroey, P. Nagy, H. Tournaye, J. Liu, S. Silber, A. van Steirteghem, Outcome of intracytoplasmic sperm injection with testicular spermatozoa in obstructive and non-obstructive azoospermia, Hum. Reprod. 11 (5) (1996) 1015–1018.
- S.J. Silber, Z. Nagy, J. Liu, H. Tournay, W. Lissens, C. Ferec, I. Liebaers, P. Devroey, A.C. van Steirteghem, The use of epididymal and testicular spermatozoa for intracytoplasmic sperm injection: the genetic implications for male infertility, Hum. Reprod. 10 (8) (1995) 2031–2043. [94] S.J. Silber, Z.P. Nagy, J. Liu, H. Godoy, P. Devroey, A.C. van Steirteghem, Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration, Hum. Reprod. 9 (9) (1994) 1705–1709.
- S.J. Silber, A.C. van Steirteghem, J. Liu, Z. Nagy, H. Tournaye, P. Devroey, High fertilization and pregnancy rate after intracytoplasmic sperm injection with spermatozoa obtained from testicle biopsy, Hum. Reprod. 10 (1) (1995) 148–152.
- S.J. Silber, P. Devroey, H. Tournaye, A.C. van Steirteghem, Fertilizing capacity of epididymal and testicular sperm using intracytoplasmic sperm injection (ICSI), Reprod. Fertil. Dev. 7 (2) (1995) 281–293.
- S.J. Silber, A. van Steirteghem, Z. Nagy, J. Liu, H. Tournaye, P. Devroey, Normal pregnancies resulting from testicular sperm extraction and intracytoplasmic sperm injection for azoospermia due to maturation arrest, Fertil. Steril. 66 (1) (1996) 110–117.
- R. Beharka, D. Pacik, I. Crha, Long-tem experience with MESA, TESE techniques in the University Hospital (FN) in Brno, Rozhl. Chir. 85 (10) (2006) 526–529 (Slovak).
- T. Diemer, A. Hauptmann, W. Weidner, Treatment of azoospermia: surgical sperm retrieval (MESA, TESE, micro-TESE), Urologe A 50 (1) (2011) 38–46 (German).
- S.J. Silber, L.J. Rodriguez-Rigau, Quantitative analysis of testicle biopsy: determination of partial obstruction and prediction of sperm count after surgery for obstruction, Fertil. Steril. 36 (4) (1981) 480–485.
- S.J. Silber, P. Patrizio, R.H. Asch, Quantitative evaluation of spermatogenesis by testicular histology in men with congenital absence of the vas deferens undergoing epididymal sperm aspiration, Hum. Reprod. 5 (1) (1990) 89–93.
- S.J. Silber, Microsurgical TESE and the distribution of spermatogenesis in non-obstructive azoospermia, Hum. Reprod. 15 (11) (2000) 2278–2284.
- A.C. van Steirteghem, Z. Nagy, H. Joris, J. Liu, C. Staessen, J. Smitz, A. Wisanto, P. Devroey, High fertilization and implantation rates after intracytoplasmic sperm injection, Hum. Reprod. 8 (7) (1993) 1061–1066.
- S.J. Silber, A modern view of male infertility, Reprod. Fertil. Dev. 6 (1) (1994) 93–104.
- S.J. Silber, T. Ord, C. Borrero, J. Balmaceda, R.H. Asch, New treatment for infertility due to congenital absence of the vas deferens, Lancet (1987) 850–851.
- S.J. Silber, The use of epididymal sperm for the treatment of male infertility, Baillieres Clin. Obstet. Gynaecol. 11 (4) (1997) 739–752.
- S.J. Silber, Pregnancy caused by sperm from vasa efferentia, Fertil. Steril. 49 (2) (1988) 373–375.
- S.J. Silber, T. Ord, J. Balmaceda, P. Patrizio, R. Asch, Congenital absence of the vas deferens. The fertilizing capacity of human epididymal sperm, N. Engl. J. Med. 323 (26) (1990) 1788–1792.
- H. Tournaye, J. Liu, P.Z. Nagy, M. Camus, A. Goossens, S. Silber, A.C. Van Steirteghem, P. Devroey, Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa, Hum. Reprod. 11 (1) (1996) 127–132.
- H. Tournaye, G. Verheyen, P. Nagy, F. Ubaldi, A. Goossens, S. Silber, A.C. Van Steirteghem, P. Devroey, Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum. Reprod. 12 (1) (1997) 80–86.
- S. Friedler, A. Raziel, D. Strassburger, Y. Soffer, D. Komarovsky, R. Ron-El, Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia, Hum. Reprod. 12 (7) (1997) 1488–1493.
- B. Rosenlund, U. Kvst, L. Ploen, B.L. Rozell, P. Sjoblom, T. Hillensjo, A comparison between open and percutaneous needle biopsies in men with azoospermia, Hum. Reprod. 13 (5) (1998) 1266–1271.
- S.J. Silber, L. Johnson, G. Verheyen, A. van Steirteghem, Round spermatid injection, Fertil. Steril. 73 (5) (2000) 897–900.
- S.J. Silber, New concepts in operative andrology: a review, Int. J. Androl. 23 (Suppl. 2) (2000) 66–76.
- In: A.F. Holstein, E.D. Roosen-Runge (Eds.), Atlas of Human Spermatogenesis, Grosse Verlag, Berlin, 1981.
- P. Schlegel, T. Chang, The testis, epididymis and ductus deferens, in: P. Walsh, A. Retic, T. Stamey, E. Vaughan (Eds.), Campbell’s Urology, 6th ed., Saunders, Philadelpha, 1991, p. 190.
- P.N. Schlegel, L.M. Su, Physiologic consequences of testicular sperm extraction, Hum. Reprod. 12 (8) (1997) 1688–1692.
- P.N. Schlegel, G.D. Palermo, M. Goldstein, S. Menendez, N. Zaninovic, L.L. Veeck, Z. Rosenwaks, Testicular sperm extraction with intracytoplasmic sperm injection for non-obstructive azoospermia, Urology 49 (3) (1997) 435–440.
- P.N. Schlegel, Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision, Hum. Reprod. 14 (1) (1999) 131–135.
- R. Reijo, T.Y. Lee, P. Salo, R. Alagappan, L.G. Brown, M. Rosenberg, S. Rozen, T. Jaffe, D. Straus, O. Hovatta, A. de la Chapelle, S. Silber, D.C. Page, Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene, Nat. Genet. 10 (4) (1995) 383–393.
- T. Kuroda-Kawaguchi, H. Skaletsky, L.G. Brown, P.J. Minx, H.S. Cordum, R.H. Waterston, R.K. Wilson, S. Silber, R. Oates, S. Rozen, D.C. Page, The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men, Nat. Genet. 29 (3) (2001) 279–286.
- S.J. Silber, R. Alagappan, L.G. Brown, D.C. Page, Y chromosome deletions in azoospermic and severely oligozoospermic men undergoing intracytoplasmic sperm injection after testicular sperm extraction, Hum. Reprod. 13 (12) (1998) 3332–3337.
- S.J. Silber, S. Repping, Transmission of male infertility to future generations: lessons from the Y chromosome, Hum. Reprod. Update 8 (3) (2002) 217–229.
- D.C. Page, S. Silber, L.G. Brown, Men with infertility caused by AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility, Hum. Reprod. 14 (7) (1999) 1722–1726.
- R. Saxena, L.G. Brown, T. Hawkins, R.K. Alagappan, H. Skaletsky, M.P. Reeve, R. Reijo, S. Rozen, M.B. Dinulos, C.M. Disteche, D.C. Page, The DAZ gene cluster on the human Y chromosome arose from an autosomal gene that was transposed, repeatedly amplified and pruned, Nat. Genet. 14 (3) (1996) 292–299.
- R. Saxena, J.W. de Vries, S. Repping, R.K. Alagappan, H. Skaletsky, L.G. Brown, P. Ma, E. Chen, J.M. Hoovers, D.C. Page, Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome, Genomics 67 (3) (2000) 256–267.
- H. Skaletsky, T. Kuroda-Kawaguchi, P.J. Minx, H.S. Cordum, L. Hillier, L.G. Brown, S. Repping, T. Pyntikova, J. Ali, T. Bieri, A. Chinwalla, A. Delehaunty, K. Delehaunty, H. Du, G. Fewell, L. Fulton, R. Fulton, T. Graves, S.F. Hou, P. Latrielle, S. Leonard, E. Mardis, R. Maupin, J. McPherson, T. Miner, W. Nash, C. Nguyen, P. Ozersky, K. Pepin, S. Rock, T. Rohlfing, K. Scott, B. Schultz, C. Strong, A. Tin-Wollam, S.P. Yang, R.H. Waterson, R.K. Wilson, S. Rozen, D.C. Page, The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes, Nature 423 (6942) (2003) 825–837.
- R.D. Oates, S. Silber, L.G. Brown, D.C. Page, Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI, Hum. Reprod. 17 (11) (2002) 2813–2824.
- S.J. Silber, D.C. Page, L.G. Brown, R. Oates, ICSI results with and without Y chromosomal deletions in men with severe oligozoospermia and azoospermia, in: 57th Annual Meeting of the ASRM, Orlando, Florida, 2001, (Abstract P-83).
- P. Devroey, J. Liu, Z. Nagy, H. Tournaye, S.J. Silber, A.C. van Steirteghem, Normal fer- tilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection, Fertil. Steril. 62 (3) (1994) 639–641.
- B.T. Lahn, D.C. Page, Functional coherence of the human Y chromosome, Science 278 (5338) (1997) 675–680.
- B.T. Lahn, D.C. Page, Retropostition of autosomal mRNA yielded testis-specific gene family on human Y chromosome, Nat. Genet. 21 (1999) 429–433.
- J. Lange, H. Skaletsky, S.K. van Daalen, S.L. Embry, C.M. Korver, L.G. Brown, R.D. Oates, S. Silber, S. Repping, D.C. Page, Isodicentric Y chromosomes and sex disorders as byproducts of homologous recombination that maintains palindromes, Cell 138 (5) (2009) 855–869.
- L. Tiepolo, O. Zuffardi, Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome on the long arm, Hum. Genet. 34 (2) (1976) 119–124.
- P.H. Vogt, A. Edelman, S. Kirsch, O. Henegariu, P. Hirschmann, F. Kiesewetter, F.M. Kohn, W.B. Schill, S. Farah, C. Ramos, M. Hartmann, W. Hartschuh, D. Meschede, H.M. Behre, A. Castel, E. Nieschlag, W. Weidner, H.-J. Grone, A. Jung, W. Engel, G. Haidl, Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11, Hum. Mol. Genet. 5 (7) (1996) 933–943.
- S.J. Qureshi, A.R. Ross, K. Ma, H.J. Cooke, M.A. Intyre, A.C. Chandley, T.B. Hargreave, Polymerase chain reaction screening for Y chromosome microdeletions: a first step toward the diagnosis of genetically-determined spermatogenic failure in men, Mol. Hum. Reprod. 2 (10) (1996) 775–779.
- J.L. Pryor, M. Kent-First, A. Muallem, A.H. van Bergen, W.E. Nolten, L. Meisner, K.P. Roberts, Microdeletions in the Y chromosome of infertile men, N. Engl. J. Med. 336 (1997) 534–540.
- G.M. Brown, R.A. Furlong, C.A. Sargent, R.P. Erickson, G. Longepied, M. Mitchell, M.H. Jones, T.B. Hargreave, H.J. Cooke, N.A. Affara, Characterisation of the coding sequence and fine mapping of the human DFFRY gene and comparative expression analysis and mapping to the Sxrb interval of the mouse Y chromosome of the Dffry gene, Hum. Mol. Genet. 7 (1) (1998) 97–107.
- C. Sargent, C. Boucher, S. Kirsch, G. Brown, B. Weiss, A. Trundley, P. Burgoyne, N. Sault, C. Durand, N. Levy, P. Terriou, T. Hargreave, H. Cooke, M. Mitchell, G. Rappold, N. Affara, The critical region of overlap definings of the AZFa male infertility interval of proximal Yq contains three transcribe sequences, J. Med. Genet. 36 (9) (1999) 670–677.
- C. Sun, H. Skaletsky, B. Birren, K. Devon, Z. Tang, S. Silber, R. Oates, D.C. Page, An azoospermic man with a de novo point mutation in the Y-chromosomal gene USP9Y, Nat. Genet. 23 (4) (1999) 429–432.
- C. Sun, H. Skaletsky, S. Rozen, J. Gromoll, E. Nieschlag, R. Oates, D.C. Page, Deletion of Azoospermia Factor a (AZFa) region of human Y chromosome cause by recombination between HERV15 proviruses, Hum. Mol. Genet. 9 (15) (2000) 2291–2296.
- S.W. Kim, K.D. Kim, J.S. Paick, Microdeletions within the azoospermia factor subregions of the Y chromosome in patients with idiopathic azoospermia, Fertil. Steril. 72 (2) (1999) 349–353.
- S. Repping, H. Skaletsky, J. Lange, S. Silber, F. van der Veen, R.D. Oates, D.C. Page, S. Rozen, Recombination between palindromes P5 to P1 on the human Y chromosome causes massive deletions and spermatogenic failure, Am. J. Hum. Genet. 71 (4) (2002) 906–922.
- R.A. Brandell, A. Mielnik, D. Liotta, Z. Ye, L.L. Veeck, G.D. Palermo, P.N. Schlegel, AZFb deletions predict the absence of spermatozoa with testicular sperm extraction: preliminary report of a prognostic genetic test, Hum. Reprod. 13 (10) (1998) 2812–2815.
- M.C. Martinez, M.J. Bernabe, E. Gomez, A. Ballesteros, J. Landeras, G. Glover, M. Gil-Salom, J. Remohi, A. Pellicer, Screening for AZF deletion in a large series of severely impaired spermatogenesis patients, J. Androl. 21 (5) (2000) 651–655.
- K. Ma, A. Sharkey, S. Kirsch, P. Vogt, R. Keil, T.B. Hargreave, S. McBeath, A.C. Chandley, Towards the molecular localisation of the AZF locus: mapping of microdeletions in azoospermic men within 14 subintervals of interval 6 of the human Y chromosome, Hum. Mol. Genet. 1 (1) (1992) 29–33.
- [147] D.J. Elliott, M.R. Millar, K. Oghene, A. Ross, F. Kiesewetter, J. Pryor, M. McIntyre, T.B. Hargreave, P.T.K. Saunders, P.H. Vogt, A.C. Chandley, H. Cooke, Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromo- some long arm, Proc. Natl. Acad. Sci. U.S.A. 94 (8) (1997) 3848–3853.
- [148] K. Ma, J.D. Inglis, A. Sharkey, W.A. Bickmore, R.E. Hill, E.J. Prosser, R.M. Speed, E.J. Thomson, M. Jobling, K. Taylor, A Y chromosome gene family with RNA-binding protein homology: candidates for the azoospermia factor AZF controlling human spermatogenesis, Cell 75 (7) (1993) 1287–1295.
- [149] S. Foote, D. Vollrath, A. Hilton, D.C. Page, The human Y chromosome: overlapping DNA clones spanning the euchromatic region, Science 258 (5079) (1992) 60–66.
- [150] K. Kobayashi, K. Mizuno, A. Hida, R. Komaki, K. Tomita, I. Matsushita, M. Namiki, T. Iwamoto, S. Tamura, S. Minowada, Y. Nakahori, PCR analysis of the Y chromosome long arm in azoospermic patients: evidence for a second locus required for spermatogenesis, Hum. Mol. Genet. 3 (11) (1994) 1965–1967.
- [151] N.N. Chai, E.C. Salido, P.H. Yen, Multiple functional copies of the RBM gene family, a spermatogenesis candidate on the human Y chromosome, Genomics 45 (2) (1997) 355–361.
- [152] N. Affara, C. Bishop, W. Brown, H. Cooke, P. Davey, N. Ellis, J.M. Graves, M. Jones, M. Mitchell, G. Rappold, C. Tyler-Smith, P. Yen, Y.F. Lau, Report of the Second International Workshop on Y Chromosome Mapping 1995, Cytogenet. Cell Genet. 73 (1–2) (1996) 33–76.
- [153] R. Reijo, R.K. Alagappan, P. Patrizio, D.C. Page, Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome, Lancet 347 (9011) (1996) 1290–1293.
- [154] D.B. Menke, G.L. Mutter, D.C. Page, Expression of DAZ, an azoospermia factor candidate, in human spermatogonia, Am. J. Hum. Genet. 60 (1) (1997) 237–241. [155] J.H. Hackstein, R. Hochstenbach, The elusive fertility genes of Drosophila: the ultimate haven for selfish genetic elements, Trends Genet. 11 (5) (1995) 195–200. [156] H.J. Cooke, M. Lee, S. Kerr, M. Ruggiu, A murine homologue of the human DAZ gene is autosomal and expressed only in male and female gonads, Hum. Mol. Genet. 5 (4) (1996) 513–516.
- [157] C.G. Eberhart, J.Z. Maines, S.A. Wasserman, Meiotic cell cycle requirement for a fly homologue of human deleted in Azoospermia, Nature 381 (6585) (1996) 783–785.
- [158] T. Karashima, A. Sugimoto, M.A. Yamamoto, A C. elegans homologue of DAZ/boule is involved in progression through meiosis during oogenesis, Worm Breed. Gaz. 15 (1) (1997) 65.
- [159] M. Ruggiu, R. Speed, M. Taggart, S.J. McKay, F. Kilanowski, P. Saunders, J. Dorin, H.J. Cooke, The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis, Nature 389 (6646) (1997) 73–77.
- [160] D.W. Houston, J. Zhang, J.Z. Maines, S.A. Wasserman, M.L. King, A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule, Development 125 (2) (1998) 171–180.
- 161] N. Saut, P. Terriou, A. Navarro, N. Levy, M.J. Mitchell, The human Y chromosome genes BPY2, CDY1 and DAZ are not essential for sustained fertility, Mol. Hum. Reprod. 6 (9) (2000) 789–793.
- [162] E. Moro, A. Ferlin, P.H. Yen, P.G. Franchi, G. Palka, C. Foresta, Male infertility caused by a de Novo partial deletion of the DAZ clusters on the Y chromosome, J. Clin. Endocrinol. Metab. 85 (11) (2000) 4069–4073.
- [163] T. Bienvenu, C. Patrat, K. McElreavey, M. de Almeida, P. Jouannet, Reduction in the DAZ gene copy number in two infertile men with impaired spermatogenesis, Ann. Genet. 44 (3) (2001) 125–128.
- [164] J.W. de Vries, M.J. Hoffer, S. Repping, J.M. Hoovers, N.J. Leschot, F. van der Veen, Reduced copy number of DAZ genes in subfertile and infertile men, Fertil. Steril. 77 (1) (2002) 68–75.
- [165] S. Repping, S.K. van Daalen, C.M. Korver, L.G. Brown, J.D. Marszalek, J. Gianotten, R.D. Oates, S. Silber, F. van der Veen, D.C. Page, S. Rozen, A family of human Y chromosomes has dispersed throughout Northern Eurasia despite a 1.8-Mb deletion in AZFc region, Genomics 83 (6) (2004) 1046–1052.
- [166] P. Blanco, M. Shlumukova, C.A. Sargent, M.A. Jobling, N. Affara, M.E. Hurles, Divergent outcomes of intrachromosomal recombination on the human Y chromosome: male infertility and recurrent polymorphism, J. Med. Genet. 37 (10) (2000) 752–758.
- [167] C. Kamp, P. Hirschmann, H. Voss, K. Huellen, P.H. Vogt, Two long homologous retroviral sequence blocks in proximal Yq11 cause AZFa microdeletions as a re- sult of intrachromosomal recombination events, Hum. Mol. Genet. 9 (17) (2000) 2563–2572.
- [168] S. Rozen, H. Skaletsky, J.D. Marszalek, P.J. Minx, H.S. Cordum, R.H. Waterston, R.K. Wilson, D.C. Page, Abundant gene conversion between arms of palindromes in human and ape Y chromosome, Nature 423 (6942) (2003) 873–876.
- [169] K. Jegalian, D.C. Page, A proposed path by which genes common to mammalian X and Y chromosomes evolve to become X inactivated, Nature 394 (6695) (1998) 776–780.
- [170] K. Jegalian, B.T. Lahn, Why the Y is so weird, Sci. Am. 284 (2001) 56–61.
- [171] B.T. Lahn, D.C. Page, Four evolutionary strata on the human X chromosome, Science 286 (5441) (1999) 964–967.
- [172] M.L. Delbridge, J.L. Harry, R. Toder, R.J. O’Neill, K. Ma, A.C. Chandley, J.A. Graves, A human candidate spermatogenesis gene, RBM1, is conserved and amplified on the marsupial Y chromosome, Nat. Genet. 15 (2) (1997) 131–136.
- [173] M.L. Delbridge, P.A. Lingenfelter, C.M. Disteche, J.A. Graves, The candidate spermatogenesis gene RBMY has a homologue on the human X chromosome, Nat. Genet. 22 (3) (1999) 223–224.
- 174] S. Mazeyrat, N. Saut, M.G. Mattei, M.J. Mitchell, RBMY evolved on the Y chromosome from a ubiquitously transcribed X-Y identical gene, Nat. Genet. 22 (3) (1999) 224–226.
- T. Vogel, R.M. Speed, P. Teague, H.J. Cooke, Mice with Y chromosome deletion and reduced Rbm genes on a heterozygous Dazl1 null background mimic a human azoospermic factor phenotype, Hum. Reprod. 14 (12) (1999) 3023–3029.
- J.A. Graves, Two uses for old SOX, Nat. Genet. 16 (1997) 114–115.
- A. Pask, J.A. Graves, Sex chromosomes and sex-determining genes: insights from marsupials and monotremes, Cell. Mol. Life Sci. 55 (6–7) (1999) 864–875.
- V.P. Vidal, M.C. Chaboissier, D.G. de Rooij, A. Schedl, Sox9 induces testis development in XX transgenic mice, Nat. Genet. 28 (3) (2001) 216–217.
- R.V. Short, Human reproduction in an evolutionary context, Ann. N. Y. Acad. Sci. 709 (1995) 416–425.
- S.J. Silber, The disappearing male, in: R. Jansen, D. Mortimer (Eds.), Towards Reproductive Certainty—Fertility & Genetics Beyond, The Parthenon Publishing roup, New York/London, 1999, pp. 499–505.
- J.F. Hughes, H. Skaletsky, T. Pyntikova, T.A. Graves, S.K.M. van Daalen, P.J. Minx, R.S. Fulton, S.D. McGrath, D.P. Locke, C. Friedman, B.J. Trask, E.R. Mardis, W.C. Warren, S. Repping, S. Rozen, R.K. Wilson, D.C. Page, Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content, Nature 463 (7280) (2010) 536–539.
- M.J. Noordam, S.K. van Daalen, S.E. Hovingh, C.M. Korver, F. van der Veen, S. Repping, A novel partial deletion of the Y chromosome azoospermia factor C region is caused by non-homologous recombination between palindromes and may be associated with increased sperm counts, Hum. Reprod. 26 (3) (2011) 713–723.
- P.J. Wang, J.R. McCarrey, F. Yang, D.C. Page, An abundance of X-linked genes expressed in spermatogonia, Nat. Genet. 27 (4) (2001) 422–426.
- R.L. Brinster, M.R. Avarbock, Germline transmission of donor haplotype following spermatogonial transplantation, Proc. Natl. Acad. Sci. U. S. A. 91 (1994) 11303–11307.
- R.L. Brinster, J.W. Zimmerman, Spermatogenesis following male germ-cell transplantation, Proc. Natl. Acad. Sci. U. S. A. 91 (1994) 11289–11302.
- M. Nagano, R.L. Brinster, Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient males, Acta Pathol. Microbiol. Immunol. Scand. 106 (1998) 47–55.
- T. Ogawa, J.M. Arechnaga, M.R. Avarbock, R.L. Brinster, Transplantation of testis germinal cells into mouse seminiferous tubules, Int. J. Dev. Biol. 41 (1) (1997) 111–122.
- M. Kanatsu-Shinohara, T. Muneto, J. Lee, M. Takenaka, S. Chuma, N. Nakatsuji, T. Horiuchi, T. Shinohara, Long term culture of male germline stem cells from hamster testes, Biol. Reprod. 78 (4) (2008) 611–617.
- M. Nagano, P. Patrizio, R.L. Brinster, Long-term survival of human spermatogonial stem cells in mouse testes, Fertil. Steril. 78 (6) (2002) 1225–1233.
- S. Schlatt, G. Rosiepen, G.F. Weinbauer, C. Rolf, P.F. Brook, E. Nieschlag, Germ cell transfer into rat, bovine, monkey and human testes, Hum. Reprod. 14 (1) (1999) 144–150.
- M.R. Avarbock, C.J. Brinster, R.L. Brinster, Reconstitution of spermatogenesis from frozen spermatogonial stem cells, Nat. Med. 2 (1996) 693–696.
- [192] H. Sadri-Ardekani, S.C. Mizrak, S.K.M. van Daalen, C.M. Korver, H.L. Roepers-Gajadien, M. Koruji, S. Hovingh, T.M. de Reijke, J.J.M.C.H. de la Rosette, F. van der Veen, D.G. de Rooij, S. Repping, A.M.M. van Pelt, Propagation of human spermatogonial stem cells in vitro, JAMA 302 (19) (2009) 2127–2134.
- [193] S.C. Mizrak, J.V. Chikhovskaya, H. Sandri-Ardekani, S. van Daalen, C.M. Korver, S.E. Hovingh, H.L. Roepers-Gajadien, A. Raya, K. Fluiter, Th.M. de Reijke, J.J.M.C.H. de la Rosette, A.C. Knegt, J.C. Belmonte, F. van der Veen, D.G. de Rooij, S. Repping, A.M.M. van Pelt, Embryonic stem cell-like cells derived from adult human testis, Hum. Reprod. 25 (1) (2010) 158–167.
- [194] A. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu, T. Murray, M.J. Thun, Cancer statistics, 2008, CA Cancer J. Clin. 58 (2) (2008) 71–96.
- [195] W.A. Bleyer, The impact of childhood cancer on the United States and the world, CA Cancer J. Clin. 40 (6) (1990) 355–367.
- [196] J. Blatt, Pregnancy outcome in long-term survivors of childhood cancer, Med. Pediatr. Oncol. 33 (1) (1999) 29–33.
- [197] H. van den Berg, S. Repping, F. van der Veen, Parental desire and acceptability of spermatogonial stem cell cryopreservation in boys with cancer, Hum. Reprod. 22 (2) (2007) 594–597.
- [198] W.H. Wallace, R.A. Anderson, D.S. Irvine, Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 6 (4) (2005) 209–218.
- [199] J.S. Jeruss, T.K. Woodruff, Preservation of fertility in patients with cancer, N. Engl. J. Med. 360 (9) (2009) 902–911.
- [200] R.L. Brinster, Male germline stem cells: from mice to men, Science 316 (5823) (2007) 404–405.
- [201] M. Nagano, M.R. Avarbock, E.B. Leonida, C.J. Brinster, R.L. Brinster, Culture of mouse spermatogonial stem cells, Tissue Cell 30 (4) (1998) 389–397.
- [202] M. Kanatsu-Shinohara, N. Ogonuki, K. Inoue, H. Miki, A. Ogura, S. Toyokuni, T. Shinohara, Long-term proliferation in culture and germline transmission of mouse male germline stem cells, Biol. Reprod. 69 (2) (2003) 612–616.
- [203] M. Kanatsu-Shinohara, N. Ogonuki, T. Iwano, J. Lee, Y. Kazuki, K. Inoue, H. Miki, M. Takehashi, S. Toyokuni, Y. Shinkai, M. Oshimura, F. Ishino, A. Ogura, T. Shinohara, Genetic and epigenetic properties of mouse male germline stem cells during long-term culture, Development 132 (18) (2005) 4155–4163.
- [204] M. Kanatsu-Shinohara, H. Miki, K. Inoue, N. Ogonuki, S. Toyokuni, A. Ogura, T. Shinohara, Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions, Biol. Reprod. 72 (4) (2005) 985–991.
- [205] F.K. Hamra, K.M. Chapman, D.M. Nguyen, A.A. Williams-Stephens, R.E. Hammer, D.L. Garbers, Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture, Proc. Natl. Acad. Sci. U.S.A. 102 (48) (2005) 17430–17435.
- [206] H. Sadri-Ardekani, M.A. Akhondi, F. van der Veen, S. Repping, A.M. van Pelt, In vitro propagation of human prepubertal spermatogonial stem cells, JAMA 305 (23) (2011) 2416–2418.


