MICROSURGICAL (TESE) TESTICULAR SPERM EXTRACTION FOR AZOOSPERMIC MEN
Abstract of Presentation for Meeting of the American Society for Reproductive Medicine in September 1999
Introduction
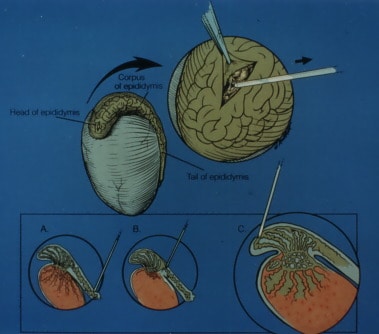
Men with non-obstructive azoospermia caused by germinal failure can now be treated successfully in many cases using testicular sperm extraction (TESE–a term which we coined in 1993) and ICSI. There is a threshold of quantitative sperm production in the deficient testis, below which no sperm will reach the ejaculate (azoospermia). This threshold phenomenon of spermatogenesis is the reason that many cases of non-obstructive azoospermia, sperm can often be extracted from testicular tissue of azoospermic men with germinal failure, and used successfully for ICSI. A prior diagnostic testicle biopsy analyzed quantitatively can often predict the likelihood of finding such sperm during a TESE-ICSI attempt.
Objectives
We wished to examine the quantitative presence of spermatogenesis in different regions of the testis in azoospermic men, in order to develop a rational microsurgical strategy for the TESE procedure. The goal was to maximize the chances for retrieving sperm from such men, to minimize tissue loss and pain, and to preserve the chance for successful future procedures.
Materials and Methods
A prospective study involving quantitative histologic analysis of testicular tissue in azoospermic men undergoing sperm retrieval for ICSI, with microsurgical removal of large contiguous areas of testicular tissue, was initiated. Careful analysis of tubular fullness observed at microsurgery was compared to quantitative histology. There were three groups. One group with non-obstructive azoospermia caused by testicular failure underwent diagnostic testicle biopsy prior to a subsequent TESE-ICSI procedure. The diagnostic testicle biopsy was analyzed quantitatively and correlated with the results of subsequent attempts at ICSI. A second group of men with non-obstructive azoospermia underwent multiple testis biopsy samplings from different regions of the testis in a testicular mapping effort during TESE. A third group of men with non-obstructive azoospermia underwent removal of large contiguous strips of testicular tissue with microsurgical dissection and evaluation of tubular dilation.
Results
Men with non-obstructive azoospermia caused by germinal failure had a mean of 0 to 3 mature spermatids per seminiferous tubule seen on a diagnostic testicle biopsy. This compared to 17 to 35 mature spermatids per tubule in men with normal spermatogenesis and obstructive azoospermia. This suggested that a certain threshold of quantitative spermatogenesis was required in order for some sperm to “spill over” into the ejaculate. In greater than half of cases, there is some sperm in the testis despite none in the ejaculate. Testicular “mapping” by multiple biopsy revealed a basically diffuse quantitative distribution of spermatogenesis. However, in 18 percent of cases undergoing multiple biopsy, there was just a rare tubule with sperm in only one (1) or two (2) out of many biopsies. This was explained in the cases that underwent microsurgical removal of contiguous strips of testicular tissue. The distribution of spermatogenesis, however sparse, was still always diffuse. A microsurgical approach resulted in the least amount of tissue loss and minimal to no pain. Under the operating microscope, tubules with no spermatogenesis were collapsed appearing, and tubules with spermatogenesis were full. This difference was apparent with Sertoli cell only, but not maturation arrest.
Conclusions
Incomplete testicular failure appears to involve a sparse, but diffuse, distribution of spermatogenesis throughout the entire testicle, rather than a patchy, or local distribution in just a few areas. This can often be observed under the operating microscope. Therefore, in most cases only one modest size biopsy is necessary. However, in cases where the distribution of sperm is sparsely diffused, a microsurgical approach is required in order to either locate tubules with spermatogenesis and remove minimal tissue, or to minimize pain and tissue loss when greater amounts of tissue must be removed in order to find sperm. Because of the diffuse distribution, a microsurgical TESE procedure, by minimizing tissue removal or secondary damage, can assure that future attempts at TESE-ICSI will not be compromised.


