Ovarian tissue vitrification—Clinical realities and outcomes
By SHERMAN SILBER
Vitrification in Assisted Reproduction, Second Edition
Edited by Michael J. Tucker, PhD, FIboil, HCLD(AAB) & Juergen Liebermann, PhD, HCLD(AAB)
Download PDF version of Dr. Silber’s Chapter
Download PDF version of the Foreword
Download PDF version of the Title Page
Preface
The developed world is in the midst of a widespread infertility epidemic. Economies in Japan, the United States, southern Europe, and even China are threatened by a decreasing population of young people having to support an increasing population of elderly people and retirees.[1] Infertility clinics are emerging throughout the world in huge numbers because of a worldwide decline in fertility as women age and become less fertile.2 In her teenage years, a woman has a 0.2% chance of being infertile, and by her early twenties, it is up to 2%. By her early thirties, it is up to 20%.[2,3] Most modern women today do not think of having a baby until their mid-thirties, and by then, over 25% are infertile, simply because of aging and the decline in the number and quality of their oocytes. This is clearly demonstrated by the high pregnancy rate via using donor oocytes from young women placed into the uteruses of older women.[2–4]
Until recently, oocyte freezing had very poor to no success, and so ovary tissue slow freezing was the only preservation method we could rely upon. Of course, now we also have a favorable option of retrieving oocytes after ovarian stimulation and egg retrieval, using vitrification instead of slow freezing for cryopreservation.[5,6]
However, as we will discuss later in this chapter, many programs are not even aware of their terrible results with oocyte freezing because they are either using a brainless commercial product, or they simply are not using the best protocol perfectly. Success with oocyte freezing should be 95%–99%, but most clinics come nowhere near this. We will explain this in this chapter, but meanwhile, we expect that there will be many unhappy “fooled” women complaining about this in the next 5–10 years. However with our approach to egg freezing as well as the option of ovary freezing we can now give women very good results for preserving their fertility against the ravages of age.
Ovarian tissue vitrification—Clinical realities and outcomes
Let us take the clinical evolution of this technology in logical order. The first successful fresh human ovary transplantation was reported between a pair of remarkable monozygotic twins discordant for premature ovarian failure (POF) using a cortical grafting technique.[7] This key event allowed us to assess the results of fresh transplantation unclouded by the confusion that might have been caused by freezing. The transplantation technique has subsequently been refined over a larger series of nine consecutive successful fresh ovary transplants in identical twins (plus two fresh allotransplants to be treated separately), with resumption of normal hormonal cycling and menstruation in all cases, eventually leading to 14 pregnancies and 11 healthy babies born from the 9 fresh identical twin recipients.[8–11] This unusual consecutive series of fresh ovary cortical transplants helped us also to refine the techniques necessary for successful preservation of fertility for cancer patients using ovarian tissue freezing, with six additional successful pregnancies from nine frozen transplants. This unusual series also helped to establish a method for distinguishing between the egg loss from transplant ischemia versus the egg loss from cryopreservation. We now can report long-term follow-up (up to 8 years) of this original series of fresh transplants, and add to it our more recent experience with cryopreserved ovarian tissue.
Micro-hematoma formation under the graft was avoided by micro-bipolar cautery and micro-pressure stitches of 9-0 nylon. Constant pulsatile irrigation with heparinized saline prevented adhesions (Figure 22.1a–d).
Vitrification in Assisted Reproduction
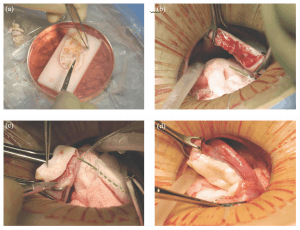
Figure 22.1: Steps in the procedure of ovarian transplantation between monozygotic (MZ) twin sisters: (a) preparation of donor ovarian cortex by dissection in a Petri dish on ice; (b) preparation of recipient ovarian medulla; (c) attaching donor cortical tissue to recipient ovarian medulla; (d) attaching thawed donor cortical tissue for retransplant to the recipient medulla.
Ovarian Cryopreservation
All of the frozen cases in the past that transplanted back into the patient utilized the slow-freeze approach.[12–14] However, we now use vitrification exclusively for cryopreservation in humans because of the results of in vitro viability analysis in humans, as well as in vivo transplant studies in the bovine and human.[11,15]
The goal of the in vitro study was to determine which method produced a higher cell survival rate: slow freeze or vitrification. The high viability (92%) of oocytes in control (fresh) specimens indicated only minimal damage to the eggs.[11] Overall, 2301 oocytes were examined from 16 specimens. Results within each of the three groups revealed no significant differences between fresh and vitrified tissue, but the viability of slow freeze-cryopreserved tissue was less than half that of vitrified tissue or controls (42%) (p < 0.01). Transmission electron microscopy has also been used to analyze ovarian tissue that had been either cryopreserved by slow freezing or vitrified by ultra- rapid freezing, showing vitrification to be superior.[16]
Standard H&E histology showed no difference between pre-freeze ovarian tissue and post-vitrification ovarian tissue (Figure 22.2a and b).
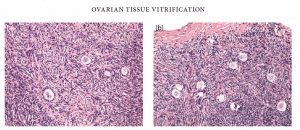
Figure 22.2: Histology (a) pre- and (b) post-vitrification of ovarian tissue
Finally, quantitative histologic study of primordial follicles in the bovine after vitrification and transplantation back to the cow 2 months later remarkably showed no follicle loss.
The basic science concept of vitrification, whether for eggs, embryos, or tissue, is to completely avoid any ice crystal formation by using a very high concentration of cryoprotectant and a very rapid rate (virtually “instant”) of cooling. This is quite different from classic slow-freeze cooling, which relies on a partial and very gradual removal of water from the cell by encouraging ice crystal formation preferentially on the outside of the cell, drawing water out.
Using the vitrification technique, cortex tissue of each ovary is cut into slices of 1 × 10 × 10 mm. Ovarian tissues are initially equilibrated in 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) in handling medium (HM; HEPES-buffered TCM-199 solution supplemented with 20% serum substitution DMSO; cat. no. D2650; Sigma Aldrich, St. Louis, MO) for 25 minutes, followed by a second equilibration in 20% EG and 20% DMSO with 0.5 mol/L sucrose for 15 minutes. Ovarian tissues are then placed in a minimum volume of solution (virtu- ally “dry”) onto a thin metal strip (Cryotissue: Kitazato BioPharma, Fujinomiya, Japan), and submerged directly into sterile liquid nitrogen,[17] following which the strip is inserted into a protective container and placed into a liquid nitrogen storage tank (Figure 22.3).
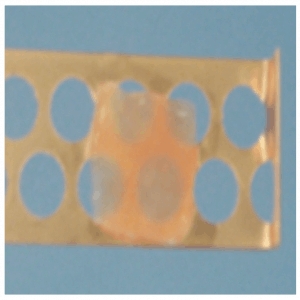
Figure 22.3: Ovarian tissue slice.
For thawing, the protective cover is removed and the Cryotissue metal strip is immersed directly into 40 mL HM solution at 37°C supplemented with 1.0 mol/L sucrose for 1 minute. Then, ovary tissues are transferred into 15 mL of 0.5 mol/L sucrose HM solution for 5 minutes at room temperature, and washed twice in HM solution for 10 minutes before viability analysis or trans- plantation. No ice crystal formation occurs during any of these vitrification procedures.[15]
One of our twin recipients became pregnant at 39 years of age without medical assistance after her fifth menses, 8 months after transplantation. She delivered a healthy baby girl at full-term, then conceived again at 42 years of age, and delivered a healthy baby boy, again at full-term, 4 years after her transplant. Her ovary is still functioning to date after 7 years, and she conceived again at 45 years of age with another healthy boy.
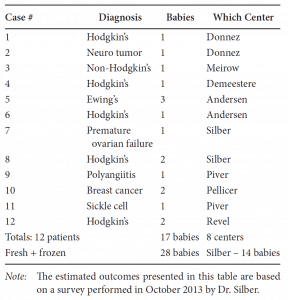
This newly favorable experience with ovarian cortex grafting is not limited just to our center.[18] Equally robust results are being experienced in Belgium, France, Spain, Denmark, and Israel. Frozen ovarian grafts (even with the slow-freeze technique) in Denmark are lasting over 5 years, and many spontaneous pregnancies have been reported with no need for in vitro fertilization (IVF) or other ancillary treatment. At the time of this writing, over 28 healthy babies have been born from ovarian tissue grafting fresh and frozen, and most involved no IVF and resulted from regular intercourse with no special treatment (Table 22.1).
Table 22.1: Worldwide Frozen Ovarian Cortex Tissue Transplant Pregnancies
Frozen Cortical Ovarian Transplantation
The most common benefit of ovarian transplantation is not the unusual cases of fresh grafting in identical twins, but rather the protection of the fertility and future endocrine function of young women undergoing cancer treatment.[11,15,19–26] Since 1996, we have fro- zen ovary tissue for 68 young women with cancer or at risk for POF, of whom 16 had spare frozen tissue subjected to detailed viability testing before cryopreservation and after thaw. Only one had ovarian metastasis, a young woman with widespread breast cancer metastasis throughout her entire body. Otherwise, none of our other 61 cases had any tumor cells in their ovaries. The reason for the remarkable absence of ovarian metastasis might possibly be due to the fibrous avascular nature of the ovarian cortex (Anderson C, personal communication). In fact, the reason why fetal ovarian tubules (which in the fetal male become seminiferous tubules) invade the fibrous cortex and become follicles is that the dense fibrous tissue of the cortex (which in the fetal and adult testis is just tunica albuginia) is needed to suppress the resting follicles from developing all at once prematurely. In addition to these 68 pathological cases, seven women have had ovarian tissue frozen simply to allow them to have the possibility of bearing children at an older age, because they had to delay childbearing for strong personal or economic reasons.
Future Prospects for Ovarian Tissue Transplantation
After ovarian transplantation, all patients were able to attempt natural conception every month without medical assistance. In fact, the commonly held view that egg freezing is a proven technique and ovary tissue transplantation is “experimental” is belied by the fact that most of the successful pregnancies resulting from fertility preservation in cancer patients thus far have been from frozen ovary tissue, and few at the date of this writing have come from frozen oocytes.[18] Of course eventually they will have long term results to report, but not yet. However, for cancer patients, ovarian tissue does have a better record currently than egg freezing. Most of our cured cancer patients who have “young” ovarian tissue frozen feel almost grateful that they had cancer, because otherwise they would share the same fear that all modern, liberated women have about their “biological clock.” At the time of this writing, we are aware of numerous other births after implanting ovarian tissue, to a total of over 37 live births thus far.[18,26–31] Thus, despite initial skepticism, this technique is now gaining worldwide acceptance and is being enthusiastically received by young women of reproductive age with cancer.
Egg and Embryo Vitrification
As mentioned in the preface, many centers that perform oocyte vitrification are not doing it well, and their protocols lead to terrible egg survival rates. Among their errors are too rapid and changing osmolality with dangerously aggressive osmotic shifts. Also, there is a common failure in failing to create a rapid enough “freeze,” and worse yet, not a rapid enough “thaw.” The commercial kits that are designed to make this “easy” often fail in this regard, and closed freezing is worse than open freezing for rapid “freeze and thaw.” Vitrification for freezing eggs or embryos was first suggested in the mid-1980s.[32,33] However, it was not until 2005 that a highly efficient method was published, which stimulated a huge wave of justified enthusiasm for this approach to egg and embryo freezing.[4,6,34,35] But the details of this successful “bridge technique” have been lost, or have given way to poor quality commercialization (Figure 22.4a–f).
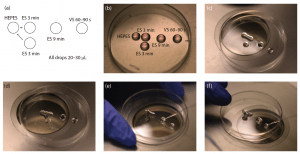
Figure 22.4: Ovarian tissue slice
The concept behind vitrification is not just its potential simplicity (given that no freezing machine is required), but that it must completely eliminate ice crystal formation. Instead of clinical IVF programs having to weigh carefully the risks to pregnancy rate posed by embryo or egg freezing, both can now be cryopreserved without concern in virtually any case in which there would be a clinical advantage. With the new vitrification methodology, there seems to be no difference between fresh and cryopreserved eggs or embryos, so long as the principles perfected in 2005 are followed.
For vitrification, the cryoprotectant solution is a combination of EG and DMSO (cat. no. D2650; Sigma Aldrich, St. Louis, MO). The embryo or egg is transferred initially into gradually increasing concentrations of equilibration solution (7.5 mol/L EG and 7.5% DMSO in 20% synthetic serum substitute [SSS]) for 10–15 minutes, followed by placement for over 1 minute in vitrification solution (15 mol/L EG and 15 mol/L DMSO in 20% SSS and 0.5 mol/L sucrose).
Contrary to popular myth, leaving it in vitrification media for less than 1 minute does not allow for enough cryoprotectant permeation. Also, this is best accomplished by the “bridge technique” to allow the most gradual increase in osmolality. The embryo is not left in a droplet, as that would slow the cooling rate. All excess fluid is removed by pipette from the Cryotop platform so that there is only a thin film of fluid surrounding it, in order to allow for the most rapid temperature drop and warming rate later. The embryo is then directly immersed into liquid nitrogen. The Cryotop containing the embryo is then placed in a canister in the liquid nitrogen tank for storage.
In the warming step, embryos are placed in decreasing concentrations of sucrose solutions to remove the cryoprotectants. The Cryotops are first rapidly plunged into a 37°C dish containing warming solution (1.0 mol/L sucrose) for 1 minute. The embryos are then slowly introduced in a stepwise fashion to dilution solutions (0.75 mol/L, 0.5 mol/L, and 0.25 mol/L sucrose). A wash solution (0.0 mol/L sucrose) is slowly added to the embryos in the dilution solution, and the final rinse for the embryos is in 100% wash solution.6 This protocol was designed to avoid too rapid osmotic shifts that could be caused by such high concentrations of cryoprotectant.
The high concentrations of cryoprotectant are actually not toxic. The appearance of toxicity comes only from too rapid an osmotic shift. The ultrarapid rate of cooling (−23,000°C/min) and the high-end concentration of cryoprotectant lowers the freezing point dramatically, and allows the ice crystallization phase to be completely avoided.
With vitrification, mature retrieved oocytes can be successfully cryopreserved with 95% success. For embryos, there really is no difference at all between fresh and frozen.[35,36]
New technology in cryopreservation via vitrification allows us to remove ovary tissue and freeze it to protect it from sterilizing cancer treatment in young women, as well as to freeze individual mature eggs. Our latest published data reveals a very high pregnancy rate with either fresh or frozen ovary tissue transplants of over 76%.37 It also allows us to stop the aging of the ovary and eggs, which is the major cause of the current worldwide infertility epidemic. It will protect the future fertility potential of young women with cancer, and will also allow an expansion of the reproductive lifespan in any young woman who wishes to delay childbearing, or delay her age of menopause.
Download PDF version of this chapter
References
- Connolly MP, Pollard MS, Hoorens S, Kaplan BR, Oskowitz SP, Silber SJ. Long-term economic benefits attributed to IVF-conceived children: A lifetime tax calculation. Am J Manag Care 2008;14(9):598–604.
- Silber SJ. How to Get Pregnant: The Classic Guide to Overcoming Infertility, 2nd ed. Little, Brown: Boston, MA, 2007.
- Mosher WD. Fecundity and infertility in the United States 1965–1982. Adv Data 1985;1:1.
- Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum Reprod Update 2012;18(1):73–91.
- Homburg R, van der Veen F, Silber SJ. Oocyte vitrification— Women’s emancipation set in stone. Fertil Steril 2009;91(4 Suppl.):1319–20.
- Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 2005;11(3):300–8.
- Silber SJ, Lenahan KM, Levine DJ et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med 2005;353(1):58–63.
- Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med 2007;356:1382–4.
- Silber SJ, DeRosa M, Pineda J et al. A series of monozygotic twins discordant for ovarian failure: Ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod 2008;23(7):1531–7.
- Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med 2008;359(24):2617–8.
- Silber SJ, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril 2010;94(6):2191–6.
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod 1994;9(4):597–603.
- Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1,2-propanediol. Hum Reprod 1999;14(8):2061–8.
- Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod 1996;11(7):1487–91.
- Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online 2009;18(4):568–77. K23395_C022.indd 195 20-08-2015 17:08:42 196 vitrification in assisted reproduction
- Keros V, Xella S, Hultenby K et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod 2009;24(7):1670–83.
- Fuentes F, Dubettier R. Air separation method and plant, United States Patent. 2004 Patent No. US 6,776,005 B2.
- Donnez J, Silber S, Andersen CY et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med 2011;43(6):437–50.
- Bleyer WA. The impact of childhood cancer on the United States and the world. CA Cancer J Clin 1990;40(6):355–67.
- Ries LAG, Smith MA, Gurney JG et al. (eds.). Cancer Incidence and Survival among Children and Adolescents: United States SEER Program, 1976–1995. National Cancer Institute, Bethesda, Maryland, 1999.
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med 2009;360(9): 902–11.
- Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 2006;21(10):2583–92.
- Anderson RA, Cameron DA. Assessment of the effect of chemotherapy on ovarian function in women with breast cancer. J Clin Oncol 2007;25(12):1630–1. Author reply 1632.
- Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in longterm survivors of radiation- and chemotherapytreated childhood cancer. J Clin Endocrinol Metab 2003;88(11):5307–14.
- Lee SJ, Schover LR, Partridge AH et al.; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24(18):2917–31.
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Anderson CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 2013;93:1503–13.
- Donnez J, Dolmans MM, Demylle D et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004;364(9443):1405–10.
- Meirow D, Levron J, Eldar-Geva T et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005;353(3):318–21.
- Andersen CY, Rosendahl M, Byskov AG et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod 2008;23(10):2266–72.
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: Successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist 2007;12(12):1437–42.
- Sanchez-Serrano M, Crespo J, Mirabet V et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril 2010;93(1):268. e11–3.
- Piver P, Amiot C, Agnani G et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. Hum Reprod 2009;24(15):10–35.
- Rall WF. Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology 1987;24(5):387–402.
- Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology 1984;21(4):407–26.
- Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology 2007;67(1):73–80.
- Katayama KP, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil Steril 2003;80(1):223–4.
- Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. RBMO 2005;30:643–50.
See also:
- “Fertility: stop all the clocks” from The London Daily Telegraph Sunday Magazine
- A Special Message From Dr. Silber About Your Biological Clock and Preserving Your Fertility
- Preserving Your Fertility
- Fertility Preservation for Cancer Patients
- Fertility expert Dr. Sherman Silber gives hope to infertile men and women
- Ovarian Tissue Freezing
- Egg, Embryo, and Sperm Freezing
- In Vitro Fertilization (IVF)
- Freezing the Biological Clock
- Complex Surgery Involved In First Successful Whole Ovary Transplant [Article]
- Giving An Ovary To My Twin Was A ‘Magical’ Opportunity [Article]
