
Hisao Osadaa,*, Sherman Silberb, Toshiyuki Kakinumaa, Masaji Nagaishia, Keiichi Katoc, Osamu Katoc
a Nihon University School of Medicine, Obstetrics and Gynaecology, Tokyo 101-8309, Japan; b Infertility Center of St. Louis at St. Luke’s Hospital, St. Louis, MO 63017, USA; c Kato Ladies Clinic, Tokyo 160-0023, Japan. * Corresponding author.
Reproductive BioMedicine Online
September, 2010
Download PDF version of this article
Abstract
The treatment for severe adenomyosis has usually been hysterectomy, because there is no line of demarcation between diseased and normal tissue. Yet many such women wish to retain their uterus and some even wish to bear children. This report evaluates the efficacy of a new method of adenomyomectomy, where adenomyotic tissues are radically excised and the uterine wall is reconstructed by a triple-flap method, without overlapping suture lines, to prevent uterine rupture in subsequent pregnancies. This is a prospective case series followed for 10 years from June 1998 to August 2008 of 104 women with severe adenomyosis verified histologically and with magnetic resonance imaging. There was a dramatic reduction in both dysmenorrhoea and hypermenorrhoea and all patients returned to having normal menstrual cycles. Of 26 women who wished to conceive, 16 became pregnant, 14 (53.8%) went to term and delivered a healthy baby and there were no cases of uterine rupture. Adenomyosis symptoms recurred in only four out of 104 cases. The procedure thus resulted in a dramatic reduction in symptoms and allowed over half of women who wished to RBMO conceive to go to term without uterine rupture.
Dr. Hisao Osada, born 1944, trained at the Department of Obstetrics and Gynaecology, Nihon University School of Medicine, Tokyo, where he later served as Professor until 2009. During 1979–1981, he trained at Mainz University, Germany and is fluent in Japanese, German and English. His specialization includes laparoscopic surgery for reproductive medicine. Dr. Osada currently teaches and practises at Nihon University and Kato Ladies Clinic, Tokyo. He is Vice President of International Association of Private Assisted Reproductive Technology Clinics and Laboratories and Vice Congress President of the 16th World Congress on In Vitro Fertilization to be held in Tokyo in 2011.

Dr. Hisao Osada, born 1944, trained at the Department of Obstetrics and Gynaecology, Nihon University School of Medicine, Tokyo, where he later served as Professor until 2009. During 1979–1981, he trained at Mainz University, Germany and is fluent in Japanese, German and English. His specialization includes laparoscopic surgery for reproductive medicine. Dr. Osada currently teaches and practises at Nihon University and Kato Ladies Clinic, Tokyo. He is Vice President of International Association of Private Assisted Reproductive Technology Clinics and Laboratories and Vice Congress President of the 16th World Congress on In Vitro Fertilization to be held in Tokyo in 2011.

Dr. Sherman Silber, a renowned pioneer in microsurgery and infertility, is considered one of the world’s leading authorities on IVF, sperm retrieval, ICSI, vasectomy reversal, tubal ligation reversal, egg and embryo freezing, ovary transplantation, and the reproductive biological clock. He performed the world’s first microsurgical vasectomy reversal, as well as the first testicle transplant, in the 70’s and now in the current century, the world’s first ovary transplant. He was the first to develop the TESE and MESA techniques for retrieving testicular and epididymal sperm in azoospermic men. He headed the clinical.
MIT team that first mapped and sequenced the Y chromosome in infertile men and discovered the now famous DAZ gene for male fertility. His research includes also the study of reproduction and fertility in zoo animals and endangered species. Most recently he has perfected the preservation of fertility for cancer patients with ovarian freezing and transplantation and thereby figured out how to extend the reproductive biological clock of women. He has helped pioneer minimal ovarian stimulation to reduce IVF costs. He has even recently answered the age-old question of why the dinosaurs went extinct by extending his research on male infertility and the Y chromosome, discovering that the change in earth temperature 65,000,000 years ago led to the birth of a skewed male/female sex ratio.
Introduction
Severe adenomyosis causes infertility, severe dysmenorrhoea and hypermenorrhoea. Since Hyama (1952) reported the use of conservative surgery for this condition, many surgical techniques have been proposed (Takeuchi et al., 2006). All of these include wedge resection of the adenomyosis followed by reconstruction of the uterine wall.
However, this approach is associated with a frequent recurrence of adenomyosis and spontaneous uterine rupture in pregnancy (Wada et al., 2006). Effective treatment requires more radical resection of the affected tissues. However, this may result in creating large defects in the uterine wall, making the reconstructed uterus incapable of sustaining a normal pregnancy. Therefore, the usual treatment for women with severe or disabling adenomyosis is hysterectomy. Yet many of these women do not want a hysterectomy and wish to carry a child. The sometime-suggested alternative of gonadotrophin-releasing hormone agonist for 3–6 months followed by IVF has many side effects and poor results in cases of massive adenomyosis. Other women, such as in the Japanese population, usually do not wish to part with their uterus for emotional or cultural reasons, but do want relief of symptoms. To overcome this difficulty and enable the uterus to sustain a pregnancy, a method of radical resection of adenomyomatous tissue along with a triple-flap method for reconstructing the uterine wall has been designed and, after obtaining approval from the ethics committee of the study centres, used in the prospective case series reported here.
Materials and methods
Between June 1998 and August 2008, radical resection of adenomyomatous tissue along with a triple-flap method for reconstructing the uterine wall was used for 104 consecutive patients with severe adenomyosis in the Department of Obstetrics and Gynecology of the Nihon University Hospital in Tokyo and at St Luke’s Hospital in St Louis in the USA. All of the 104 patients had adenomyosis involving more than 80% of the anterior and/or posterior wall of the uterus with an enlargement of more than 6 cm in thickness verified by magnetic resonance imaging (MRI) and ultrasound and confirmed by histological evaluation (Figure 1) and had been experiencing severe dysmenorrhoea and hypermenorrhoea requiring more than 5 days of analgesic drugs and/or incapacitation which necessitated over 2 days of bed rest every month. If there were less than 6 days of analgesic drugs or less than 3 days of incapacitating pain, the study centre did not operate unless the patient wanted to try to get pregnant. The infertility indication was based on size and extent of lesion as defined above. There were 38 anterior (36.5%) cases, 44 posterior (42.3%) cases and 22 (21.2%) cases that involved both anterior and posterior sides of the uterus. All women were under 45 years of age and wished to retain their uterus either for emotional or cultural reasons or, for some, to carry a baby. Post-operatively, 26 of them attempted to conceive.
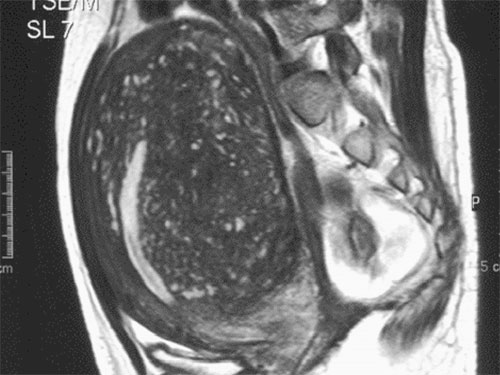
The diagnosis of the severe adenomyosis as defined above was confirmed in all 104 with transvaginal sonography with colour Doppler imaging, MRI and detailed histological evaluation after surgery. Fifty-six patients had had the history of 8 ± 5.4 (mean ± SD) years of infertility treatment. Of those, 45 (80.4%) had undergone IVF/embryo transfer (ET) and as a result 17 (37.8%) had conceived but miscarried (longest 14 weeks). Eleven patients had had the history of non-IVF treatment, such as artificial insemination with husband’s spermatozoa and ovarian stimulation, and three of these patients had conceived but miscarried by 8 weeks of pregnancy. MRI was used pre-operatively to determine thesite and the extent of adenomyosis and post-operatively to determine that blood flow had returned to the operated area. Endovaginal ultrasonography with colour Doppler imaging (EUB-650; Hitachi Medical, Tokyo, Japan) was also used to monitor the returned blood flow. The aim was to determine whether the blood flow was returned or not and the actual blood flow volume was not measured.
Surgical procedure
The surgical procedure consists of radical excision of adenomyosis (leaving a 1 cm margin of tissue above the endometrium and a 1 cm margin of tissue below the serosal surface), with subsequent triple-flap reconstruction of the uterus. A small transverse suprapubic incision is made to access the peritoneal cavity. The length of the incision is dependent on the size of the uterus, since it is lifted out of the abdomen to excise the adenomyosis and reconstruct the uterine wall. Once the uterus is extra-peritonealized, rubber tubing of approximately 6 mm width is placed widely around the proximal cervix, thus encompassing the uterine vessels as a tourniquet to prevent bleeding during the procedure (Figure 2). The placement of this tourniquet is critical to the success of the procedure. After reconstruction with the triple-flap, the tourniquet can be removed because the overlapping flaps then prevent further bleeding.
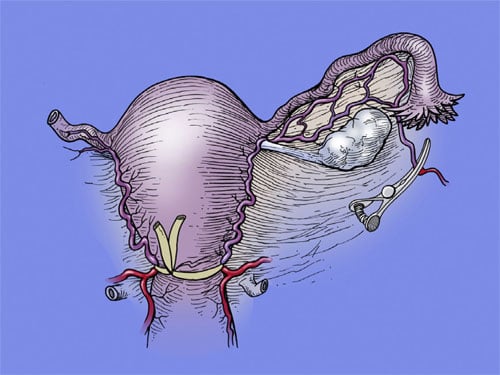
tourniquet.
After the tourniquet is in place, the surgical technique is as follows. The enlarged uterus is bisected with a scalpel from the serosal surface of the fundus, in the midline and in the sagittal plane, all the way down through the adenomyosis until the uterine cavity is reached (Figure 3A). In this way the entire extent of the adenomyosis is clearly visible, with the crucial landmarks of the endometrium and the serosal surface always in clear view. The endometrial cavity is opened sufficiently to permit the introduction of the index finger to protect and help guide during excision of the adenomyotic tissues. The adenomyotic tissues are grasped with Martin forceps and excised from surrounding myometrium leaving a myometrial thickness, from the serosa above and the endometrium below, of 1 cm. Care is also taken to avoid damage to the Fallopian tubes.
Removal of the affected tissues results in an external uterine wall composed of serosa and also 1 cm of myometrium and an inner uterine wall composed of the same thickness of myometrium and normal endometrial lining (Figure 3B and C). Of course, there is always a very small residual of adenomyosis, since there is no capsule or tissue plane as in myoma. But this layer of adenomyomatous residual tissue is soft and not problematic once the huge bulk of diseased tissue has been removed. The endometrial lining is then approximated with interrupted sutures of 3–0 Vicryl (Figure 3D). Thereafter, the myometrial defect has to be closed with the triple-flap overlap method, with care being taken to avoid overlapping suture lines.
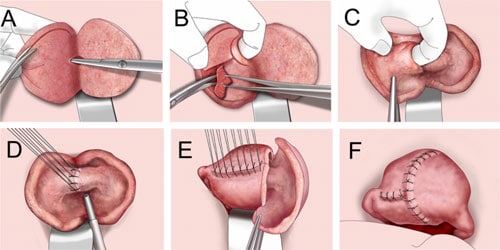
The uterus is reconstructed in the following manner. On one side of the bisected uterus the myometrium and serosa are approximated in the antero-posterior plane with many interrupted sutures of 2–0 Vicryl (Figure 3E). Then the contralateral side of the uterine wall (composed also of serosa and myometrium) is brought over the reconstructed first side in such a way as to cover the seromuscular suture line (Figure 3F). Suture lines must not overlap; only myometrial tissue flaps overlap. To accomplish this, the serosal surface of the underlying flaps must first be stripped, that is, the myometrium of the underlying flap must be denuded of serosa. Once the uterine reconstruction is complete, the rubber tubing tourniquet previously placed around the proximal cervix is removed. Remarkably, there is no significant bleeding because of the tissue pressure created by the reconstruction. The blood loss during surgery was calculated by adding the amount of blood sucked by the blood suction apparatus to the amount absorbed by the gauze. The abdominal incision is then closed in layers.
Post-operatively, after discharge from the hospital, the patients were initially followed monthly for 6 months and subsequently every 2–3 months. For the first 6 months, the uterine blood flow was checked monthly with endovaginal ultrasonography with colour Doppler imaging and with contrast-enhanced MRI every 3 months. After the initial 6 months, the assessment with an endovaginal ultrasonography with colour Doppler imaging was carried out every other month and that with MRI every 3 months. Uterine blood flow in the operated area generally returned to normal within 6 months and post-operative MRI appeared remarkably normal.
The post-operative clinical evaluation included use of the visual analogue scale (VAS) to assess the degree of hypermenorrhoea and dysmenorrhoea, initially every 3 months for the first year and thereafter 24 months post-operatively. To assess the level of menstrual bleeding, a system similar to VAS was used. The patients were asked to rate the amount of post-operative menstrual flow against that of the pre-operative level considered as 10. This permitted a more accurate semi-quantitative comparison with the pre-operative levels. The extent of dysmenorrhoea was similarly assessed using a VAS scale, also comparing the post-operative level to that of the pre-surgical level rated as 10. The reason VAS was not used in such a way as to measure bleeding and pain before the procedure was because it was thought that the patients would find it very difficult to rate the level of the pain using VAS before the operation, as it is so arbitrary what kind of pain (or blood flow) they should rate as maximum. So, the easiest and the clearest way to judge the level of pain alleviation and reduction of menstrual blood flow was to have the patient rate the pre-operative pain or blood flow level at 10 before the operation and then have them rate again after the operation. So this was a modified VAS. Student’s unpaired t-test was used to compare difference between pre-surgical and post-surgical levels of dysmenorrhoea/hypermenorrhoea and a P-value <0.01 were considered significant.
Results
All patients observed immediate relief of symptoms of hypermenorrhoea and dysmenorrhoea and their postoperative course was generally unremarkable.
The procedure was performed on 104 patients during the period from June 1998 to August 2008. The mean age of the patients in the series was 37.6 years. All candidates for surgery suffered from severe dysmenorrhoea and hypermenorrhoea and 94 women (90.4%) were found to be anaemic.
The time required to perform these procedures was (mean ± SD) 182.7 ± 62.2 min, the volume of blood loss during surgery was 372.0 ± 314.4 ml, and the weight of the excised tissues was 292.6 ± 254.1 g.
The results of the post-operative examination using contrast-enhanced MRI or an endovaginal ultrasonography with colour Doppler imaging showed that the blood flow in the operated area had returned to normal within 6 months in almost all cases (99/104, 95.2%). However, in a few cases (5/104, 4.8%) the blood flow took nearly a year to return to normal.
Post-operative complications were observed in six cases (5.8%); they were all small haematomas, under 1 cm in diameter, formed at the operated area and were all spontaneously absorbed within 2 months. There were no suture diastases (failures), infections or uterine cavity adhesions observed.
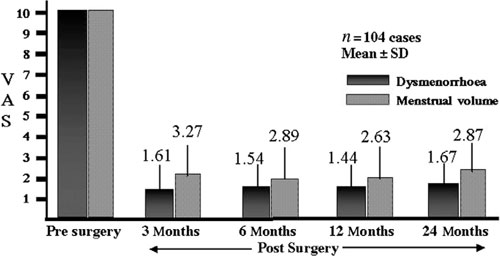
The VAS findings (mean ± SD) for dysmenorrhoea, based on a score of 10 pre-surgically, were 1.61 ± 1.43 at 3 months, 1.54 ± 1.62 at 6 months, 1.44 ± 1.65 at 1 year and 1.67 ± 1.79 at 2 years post surgery. The VAS findings for hypermenorrhoea were 3.27 ± 2.17 at 3 months, 2.89 ± 1.77 at 6 months, 2.63 ± 1.3 at 1 year and 2.87 ± 1.77 at 2 years post surgery (Figure 4). Differences between the VAS scores for pre-surgical and post-surgical dysmenorrhoea and hypermenorrhoea were statistically significant (P < 0.01 determined by Student’s unpaired t-test). There was a recurrence (defined by return of pre-op symptoms and recurrent adenomyomatous growth) in only four cases (3.8%) over the 10-year time of the study.
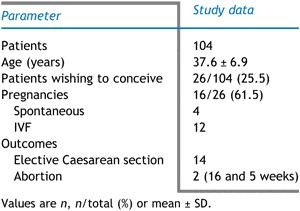
The outcome of the surgical treatment is shown in Table 1. Twenty-six women (25.0%) wished to conceive following the surgical removal of the adenomyosis. Their age (mean ± SD) was 36.9 ± 4.7 years. Sixteen of them (61.5%, 16/26) subsequently conceived. Of these, four women conceived spontaneously and 12 women conceived by IVF/ET. Two women who had IVF/ET experienced a spontaneous abortion (at 5 weeks and 16 weeks); 14 went to term and all were delivered by elective Caesarean section. There were no cases of uterine complications to the pregnancies.
Discussion
Adenomyosis may be described as a diffuse invasion of endometrial elements into the uterine myometrium. Adenomyosis differs from fibromyomatous growths in that there is no discrete border between the normal uterine tissue and the lesion, making it difficult to establish a clear dissection plane. This makes the procedure very challenging.
In addition to infertility, severe adenomyosis, also causes severe dysmenorrhoea and hypermenorrhoea, which adversely affects the woman’s well being. Management for the latter two symptoms includes long-term hormonal therapy, analgesics or, finally, hysterectomy. For those who wish to preserve reproductive functions, the surgical management of severe cases of adenomyosis is particularly difficult because one must excise diffusely involved tissue and prevent the occurrence of uterine rupture in the event of pregnancy.
Uterine rupture can occur as a complication to the enucleation of myoma or adenomyoma (Bujold et al., 2002; Guise et al., 2004; Hockstein, 2000; Landon et al., 2004; Ofir et al., 2004). Dubuisson et al. (2000) experienced three ruptures following laparoscopic myomectomies, which amounts to an incidence of 1% in their series. It is difficult to know the exact incidence of uterine rupture after adenomomectomies, since extensive conservative surgery for this condition is a relatively recent development and there has been very little literature published on the topic. However, the probability of uterine rupture after such a radical surgical intervention would likely be higher than that for myomectomy, which underlines the importance of proper reconstruction of the uterine wall.
The prerequisites for adenomyosis surgery for the purpose of preserving reproductive functions are as follows. First, it is ideal if tubal patency can be retained to allow for natural pregnancy. Second, the uterine cavity must be retained intact in order to assure implantation. Third, the uterine wall must be properly reconstructed to enable it to sustain fetal growth following conception. In other words, one must reconstruct a uterine wall which can endure the thinning associated with the expansion of the uterine cavity resulting from the development of pregnancy. There is also the problem of recurrence of the condition. However, only four (3.8%) cases of recurrence of adenomyosis were observed in this series. The patients had no further complications. Suture diastases, post-operative infections and uterine cavity adhesions were not observed.
A healing period of contraception following surgery of 6 months is suggested, although one woman conceived twins at 5 months following the surgery, went to term and was delivered by elective Caesarean section. Even women (the majority) who chose not to attempt conception were glad for personal and cultural reasons to retain their uterus and yet be relieved of their disabling symptoms.
The question might arise as to the possible use of laparoscopy instead of mini-laparotomy. Despite the first author’s extensive experience in over 5,000 cases of laparoscopic reconstruction for other conditions, the only use of laparoscopy for this procedure is to lyse adhesions prior to mini-laparotomy so as to allow the open laparotomy incision to be a relatively small one.
In conclusion, the triple-flap method offers the following advantages. First, it permits the excision of the affected tissues more widely and thoroughly than the conventional wedge resection. As a result, it appears to be extremely effective for the management of dysmenorrhoea and hypermenorrhoea. Secondly, the massive tissue defects created by the wide excision of the lesion can be reconstructed into a uterine wall of adequate thickness by the three layers of myometrium in the reconstructed wall, making the reconstructed uterus more capable of sustaining a normal pregnancy without the risk of uterine rupture. Thirdly, severe post-surgical complications were not encountered. Although recurrence of the condition may occur because adenomyosis is a progressive disorder, this series had only four such cases. Even if recurrence does occur, this technique will at least temporarily alleviate the patient’s clinical symptoms, including severe hypermenorrhoea and give her a chance to become pregnant.
Acknowledgements
The authors would like to express their gratitude to Professor Victor Gomel, MD for his kind advice and support in writing this paper and to Sharon Fuller and Kyoko Nakazato for their assistance in preparation of the manuscript.
References
- Bujold, E., Mehta, S.H., Gujold, C., Gauthier, R.J., 2002. Interdelivery interval and uterine rupture. Am. J. Obstet. Gynecol. 187, 1199–1202.
- Dubuisson, J.B., Fauconnier, A., Deffarges, J.V., Norgaard, C., Kreiker, G., Chapron, C., 2000. Pregnancy outcome and deliveries following laparoscopic myomectomy. Hum. Reprod. 15, 869–873.
- Guise, J.M., McDonagh, M.S., Osterweil, P., Nygren, P., Chan, B.K., Helfand, M., 2004. Systematic review of the incidence and consequences of uterine rupture in women with previous Caesarean section. BMJ 329, 19–25.
- Hockstein, S., 2000. Spontaneous uterine rupture in the early third trimester after laparoscopically assisted myomectomy. J. Reprod. Med. 45, 139–141.
- Hyama, L.L., 1952. Adenomyosis; its conservative surgical treatment in young women. NY State J. M., 2784.
- Landon, M.B., Hauth, J.C., Leveno, K.J., et al., 2004. National Institute of Child Health and Human Development Materna– Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior Cesarean delivery. N. Engl. J. Med. 351, 2581–2589.
- Ofir, K., Sheiner, E., Levy, A., Katz, M., Mazor, M., 2004. Uterine rupture: differences between a scarred and an unscarred uterus. Am. J. Obstet. Gynecol. 191, 425–429.
- Takeuchi, H., Kitade, M., Kikuchi, I., et al., 2006. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J. Minim. Invasiv. Gynecol. 13, 150–154.
- Wada, S., Kudo, M., Minakami, H., 2006. Spontaneous uterine rupture of a twin pregnancy after a laparoscopic adenomyomectomy: a case report. J. Minim. Invasiv. Gynecol. 13, 166–168.
Declaration: The authors report no financial or commercial conflicts of interest.


