By: Erica C. Pandolfi, Enrique Sosa, Timothy J. Hunt, Sierra Goldsmith, Kellie Hurlbut, Sherman J. Silber, Amander T. Clark
Abstract
Six human induced pluripotent stem cell sublines (hiPSCs) were generated from human dermal fibroblasts (HDFs) derived from skin biopsies donated from monozygotic twin women wherein one woman had proven fertility and her sister was infertile due to ovarian failure. Three hiPSC sublines were created from each twin’s HDFs. hiPSCs were reprogrammed using Sendai virus vectors and were subsequently positive for markers of self-renewal including OCT4, NANOG, TRA-1-81 and SSEA-4. Pluripotency was further verified using PluriTest. We show here that the hiPSC lines created from the twins are equivalent in measures of pluripotency and self-renewal, despite their differential diagnosis.
Resource Table:
| Unique stem cell lines identifier | UCLAi002-A |
| UCLAi002-B | |
| UCLAi002-C | |
| UCLAi003-A | |
| UCLAi003-B | |
| UCLAi003-C | |
| Alternative names of stem cell lines | MZT01E |
| MZT01F | |
| MZT01N | |
| MZT02D | |
| MZT02G | |
| MZT02H | |
| Institution | UCLA |
| Contact information of distributor | Dr. Amander Clark |
| Type of cell lines | hiPSC |
| Origin | Human |
| Cell Source | Fibroblasts |
| Clonality | Clonal |
| Method of reprogramming | Sendai |
| Multiline rationale | Isogenic clones |
| Gene modification | No |
| Type of modification | N/A |
| Associated disease | Ovarian failure, also known as Primary Ovarian Insufficiency (POI) |
| Gene/locus | N/A |
| Method of modification | N/A |
| Name of transgene or resistance | N/A |
| Inducible/constitutive system | N/A |
| Date archived/stock date | N/A |
| Cell line repository/bank | N/A |
| Ethical approval | UCLA Office of the Human Resource Protection Program- IRB#16-001176-CR and UCLA Embryonic Stem Cell Research Oversight Committee (ESCRO# 2016-003) |
1. Resource utility
Infertility is a condition that affects 12% of the world’s reproductive age population. Here, we generated hiPSC sublines derived from female monozygotic twins where one twin received a diagnosis of ovarian failure and infertility whereas her twin sister was fertile. Using short tandem repeat analysis (STR), the human dermal fibroblasts (HDFs) from the twin sisters were confirmed to be genetically identical. In addition, the hiPSC sublines created here are consented to be responsibly and ethically used for human fertility and infertility research.
Resource Details
One of the challenges with hiPSC studies is that genetic variability exists between unrelated individuals. The advantage of working with monozygotic twins discordant for disease is that the twin pair can serve as case-control studies. For this resource, we created a total of six independent hiPSC sublines from monozygotic twin sisters discordant for ovarian failure, and compared pluripotency and self-renewal properties across the sublines. The three hiPSC sublines created from each twin (six total hiPSC sublines) were similar in their pluripotent and self-renewal characteristics, and are therefore ideal tools for studies of ovarian failure using hiPSCs. Furthermore, these lines are consented for research in the reproductive sciences including creation of gametes and germ cells, and broader applications that facilitate the sharing of these de-identified hiPSC lines and sublines for research purposes. We believe that obtaining both specific and broad consents from donating patients is important because the use of germ cells and gametes derived from hiPSCs for research on fertility is is significant. The hiPSC sublines created here are a complement to an earlier published resource describing hiPSC sublines derived from a skin biopsy from a previously fertile woman for reproductive science research (Pandolfi et al., 2019).
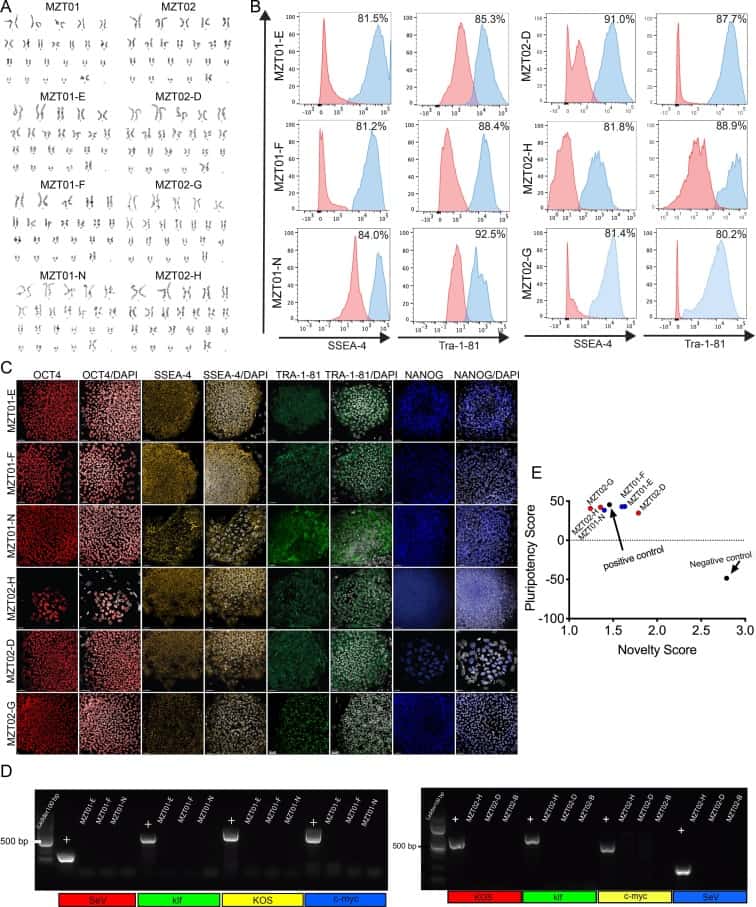
Human dermal Fibroblasts (HDFs) were derived from skin punch biopsies obtained from 39-year-old identical twin sisters. Three integration-free hiPSC sublines were generated from each twin’s HDFs using the non-integrating recombinant Sendai virus containing reprogramming factors OCT3/4, SOX2, KLF4 and c-MYC4 (Tokusumi et al., 2002). Twenty-seven days after the transduction, individual colonies were manually picked onto mouse embryonic fibroblast feeder cells to create the sublines. From the HDFs derived from the biopsy of monozygotic twin 01 (MZT01), we selected three hiPSC sublines called MZT01-E, MZT01-F and MZT01-N and characterized them for self-renewal and pluripotency (Table 1). In addition, we selected three sublines from her twin sister’s HDFs (MZT02) and called the sublines MZT02-D, MZT02-G and MZT02-H (Table 1). We first determined that the initial HDFs and the resulting hiPSC sublines were of normal 46, XX karyotype (Fig. 1A). Furthermore, all hiPSC sublines exhibited typical morphology and markers of self-renewal, as confirmed through both flow cytometry (Fig. 1B) and immunofluorescence staining for NANOG, OCT4, TRA-1-81, and SSEA-4 (Fig. 1C). Additionally, we confirmed that the reprogrammed hiPSCs did not express the exogenous reprogramming factors after continued culture (Fig. 1D) (expected band size for the positive (+) control is SeV: 181 bp, c-MYC: 532 bp, klf: 410 bp, KOS: 501 bp). To evaluate pluripotency of these lines, all hiPSC sublines were assessed using PluriTest analysis (Müller et al., 2011) (Fig. 1E). Finally, to confirm that the hiPSC sublines were of the same genetic background as the HDFs short tandem repeat (STR) analysis was conducted demonstrating that all six hiPSC lines were genetically identical to the original HDFs. Furthermore, this STR analysis confirms that the HDFs are from identical twins, as there were no discrepancies when comparing STR results from twin MZT01 with twin MZT02. We also confirmed that all hiPSC sublines were negative for mycoplasma through routine mycoplasma testing (Supplementary Fig. 1)
Table 1. Summary of lines.
| iPSC line names | Abbreviation in figures | Gender | Age | Ethnicity | Genotype of locus | Disease |
|---|---|---|---|---|---|---|
| UCLAi002-A | MZT01E | Female | 39 | unknown | N/A | POI |
| UCLAi002-B | MZT01F | Female | 39 | unknown | N/A | POI |
| UCLAi002-C | MZT01N | Female | 39 | unknown | N/A | POI |
| UCLAi003-A | MZT02D | Female | 39 | unknown | N/A | None |
| UCLAi003-B | MZT02G | Female | 39 | unknown | N/A | None |
| UCLAi003-C | MZT02H | Female | 39 | unknown | N/A | None |
2. Materials and methods
2.1. Fibroblast derivation
The 1 mm skin punch biopsies were dissected and then digested in Collagenase IV (Life Technologies) for 1 h at 37°, 5.0% CO2. The digested pieces were then plated down on 0.1% gelatin (Sigma) coated (Millipore) plates in human fibroblast media, 15% Fetal bovine serum (GE Healthcare), 1% Non-Essential Amino Acids (Invitrogen), 1% Glutamax, (GibcoTM), 1% Penicillin-Strepromyocin-Glutamine (Gibco), and Primocin (Invivogen), at 37°, 5.0% CO2. Outgrowths of human dermal fibroblasts (HDFs) were monitored for two weeks and the media was changed every three days. Fibroblasts were passaged using 0.05% Tryspin (Gibco) and re-plated, the derived cells were termed MZT01 and MZT02 based on the order the biopsy was received (Table 2 and Table 3).
Table 2. Characterization and validation.
| Classification | Test | Result | Data |
|---|---|---|---|
| Morphology | Immunofluorescence | Normal | Fig. 1 panel C |
| Phenotype | Immunofluorescence | Positive for self-renewal markers: Oct4, Nanog, SSEA-4, Tra-1–81 | Fig. 1 panel C |
| Flow cytometry | MZT04-D: Tra 1–81: 85.9%, SSEA-4: 98.7% MZT04-J: Tra 1–81: 92.7%, SSEA-4: 81.5% MZT04-C: Tra 1–81: 84.3%, SSEA-4: 81.5% |
Fig. 1 panel B | |
| Genotype | Karyotype (G-banding) and resolution | 46,XX | Fig. 1 panel A |
| Identity | Microsatellite PCR (mPCR) OR STR analysis | Performed | Supplementary Fig. 2 |
| 16 sites tested, all three lines match each other, and the HDF line they were derived from | Supplementary Fig. 2 | ||
| Mutation analysis (IF APPLICABLE) | Sequencing | N/A | |
| Southern Blot OR WGS | N/A | ||
| Microbiology and virology | Mycoplasma | Mycoplasma testing by Luminescence | Supplementary Fig. 1 |
| Differentiation potential | Pluritest | Pluripotent | Fig. 1 panel E |
| Donor screening (OPTIONAL) | N/A | ||
| Genotype additional info (OPTIONAL) | N/A | ||
| N/A |
Table 3. Reagents details.
| Antibodies used for immunocytochemistry/flow-citometry | |||
|---|---|---|---|
| Antibody | Dilution | Company Cat # and RRID | |
| Self-renewal markers | goat-anti-human Oct4 | 1:100 | Santa Cruz, sc8628 RRID: AB_653551 |
| Self-renewal markers | goat-anti-human NANOG | 1:40 | R&D Systems, AF1997 RRID: AB_355097 |
| Self-renewal markers | mouse-anti-human SSEA-4 | 1:100 | Developmental Studies Hybridoma Bank, MC-813-70 RRID: AB_528477 |
| Self-renewal markers | mouse-anti-human TRA-1-81 | 1:100 | eBiosciences, 14-8883-82 RRID: AB_891614 |
| Pluripotency markers | SSEA-4-Allophycocyanin | 1:30 | R&D Systems, FAB1435A RRID: AB_494994 |
| Pluripotency markers | TRA-1-85-Phycoerythrin | 1:60 | R&D Systems, FAB3195P RRID: AB_2066683 |
| Pluripotency markers | TRA-1-81, Alexa Fluor 488 | 1:60 | Stemcell Technologies, 60065AD RRID: AB_2721032 |
| Pluripotency markers | Dapi | 1:100 | BioVision, B1098-25 RRID: AB_2336790 |
| Secondary antibodies | AF488-conjugated donkey-anti-goat | 1:200 | JacksonImmunoResearch, 705-546-147 RRID: AB_2340430 |
| Secondary antibodies | AF488-conjugated donkey-anti-mouse | 1:200 | Life Technologies, A-21131 RRID: AB_2535771 |
| Primers | ||
| Target | Forward/Reverse primer (5′-3′) | |
| Reprogramming virus | SeV | GGA TCA CTA GGT GAT ATC GAG C/ ACC AGA CAA GAG TTT AAG AGA TAT GTA TC |
| Reprogramming virus: | KOS | ATG CAC CGC TAC GAC GTG AGC GC/ ACC TTG ACA ATC CTG ATG TGG |
| Reprogramming virus: | Klf4 | TTC CTG CAT GCC AGA GGA GCC C/ AAT GTA TCG AAG GTG CTC AA |
| Reprogramming virus: | c-Myc | TAA CTG ACT AGC AGG CTT GTC G/ TCC ACA TAC AGT CCT GGA TGA TGA TG |
2.2. Reprogramming the fibroblasts
HDFs were thawed and cultivated in human fibroblast medium. When ~80% confluent, the MZT01 and MZT02 HDFs were transfected with Sendai virus (SeV) based non-integration CytoTune™ iPS Reprogramming Kit (Life Technologies) (4) according to manufacturer’s instructions. Colonies began to appear after 11 days and were picked after three weeks. Three colonies from each HDF line were manually picked and expanded onto mouse embryonic fibroblast feeder cells in hiPSC media (DMEM/F-12 (Life Technologies), 20% KSR (Life Technologies), 10 ng/mL bFGF (R & D Systems), 1% nonessential amino acids (Life Technologies), 1% Penicillin-Strepromyocin-Glutamine (Gibco), Primocin™ (Invivogen), and 0.1 mM β-mercaptoethanol (Sigma)).
2.3. Flow cytometry
Single cell suspension was obtained using 0.05% Tryspin (Gibco). hiPSCs were then resuspended in PBS with 1% BSA. Antibody incubation lasted 30 min at 4 °C with conjugated antibodies.
2.4. Karyotyping and STR analysis
The 8 samples (two HDFs and six hiPSC sublines) were karyotyped using metaphase spreads and G-banding by Cell Line Genetics (Madison, WI). Cell Line Genetics also performed Identity analysis on the 8 cell lines using the PowerPlex 16 System (cat# DC6531, Promega).
2.5. PluriTest
Cryopreserved pelleted cells were sent to Life Sciences Solutions. Transcriptional profiles of the hiPSC lines were compared to an extensive reference set. The Pluripotency Score is an indication of how strongly a model-based pluripotency signature is expressed in the samples analyzed. The Novelty Score indicates the general model fit for a given sample.[4]
2.6. Immunofluorescence staining
Immunofluorescence staining was performed by fixing the hiPSCs in 4%PFS for 15 min at room temperature, and then permeabilizing the cells with PBS plus 0.5% Triton™ X-100 (Sigma). The hiPSCs were then blocked in 10% donkey serum (Jackson Immunoresearch) for 30 min at room temperature. Cells were incubated overnight at 4 °C with primary antibodies and then were incubated in secondary antibodies for 1 h. Immunofluorescence was imaged using a Zeiss LSM 880 confocal laser-scanning microscope.
2.7. Absence of the reprogramming virus
RNA was isolated according to manufacturer’s instructions (cytotune) (Fusaki et al., 2009) from reprogrammed fibroblasts at P0 before hiPSCs were picked and cultured. cDNA was synthesized from the RNA and RT-PCR was performed using primers provided from the manufacturer (Fusaki et al., 2009).
2.8. Mycoplasma detection
Mycoplasma was regularly tested using MycoAlert kit from Lonza Catalog #LT07-318.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are appreciative of the MCDB/BSCRC Imaging Core, BSCRC Flow cytometry core, BSCRC Genomics core. We also thank Jessica Scholes, Felicia Codrea and Jeffrey Calimlim of the UCLA BSCRC FACS core. In addition, we are grateful to Tsotne Chitiashvili for conducting the mycoplasma testing and Ernesto Rojas for assisting with creating the hiPSC sublines. Dr. Erica Pandolfi is a postdoctoral fellow supported by UPLIFT: UCLA Postdocs’ Longitudinal Investment in Faculty (Award # K12 GM106996). This project was funded by the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Innovation Award (ATC), We Finally, we gratefully acknowledge funds from the LucaBella Foundation administered through the Magee Women’s Health Research Institute and Foundation to support this work.
References
FUSAKI et al., 2009N. FUSAKI, H. BAN, A. NISHIYAMA, K. SAEKI, M. HASEGAWAEfficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genomeProc. Jpn. Acad., Ser. B, 85 (2009), pp. 348-362, 10.2183/pjab.85.348
Müller et al., 2011F.-J. Müller, B.M. Schuldt, R. Williams, D. Mason, G. Altun, E.P. Papapetrou, S. Danner, J.E. Goldmann, A. Herbst, N.O. Schmidt, J.B. Aldenhoff, L.C. Laurent, J.F. LoringA bioinformatic assay for pluripotency in human cellsNat Methods, 8 (2011), pp. 315-317, 10.1038/nmeth.1580
Pandolfi et al., 2019E.C. Pandolfi, E.J. Rojas, E. Sosa, J.J. Gell, T.J. Hunt, S. Goldsmith, Y. Fan, S.J. Silber, A.T. ClarkGeneration of three human induced pluripotent stem cell sublines (MZT04D, MZT04J, MZT04C) for reproductive science researchStem Cell Res., 40 (2019), p. 101576, 10.1016/j.scr.2019.101576
Tokusumi et al., 2002T. Tokusumi, A. Iida, T. Hirata, A. Kato, Y. Nagai, M. HasegawaRecombinant Sendai viruses expressing different levels of a foreign reporter geneVirus Research, 86 (2002), pp. 33-38, 10.1016/S0168-1702(02)00047-3


