SECOND CHANCE
New Way to Extend Fertility: Freeze Tissue From Ovaries
By Sylvia Pagán Westphal
Wall Street Journal Online, April 27, 2007
Surgery Could Expand Choices for Women, But Ethics Are Debated
Stored at subzero temperatures in a laboratory at St. Luke’s Hospital in St. Louis are eight tiny strips of tissue that were once part of Amy Johnson’s ovaries.
Ms. Johnson, 34 years old, plans to keep the tissue frozen and transplant it back into her ovaries if she has a hard time getting pregnant in the future. Back inside her body, the strips would produce relatively youthful eggs — even if Ms. Johnson is in her 40s or has reached menopause.
“I call it the backup plan,” says Ms. Johnson, a part-time business consultant in Ann Arbor, Mich. She had postponed starting a family while her husband, a professor, sought tenure. Recently she became pregnant with her first child, who was conceived without medical help, but she’s glad to have the tissue on hand in case she has trouble conceiving later.
For women who want to delay child-rearing, technology is presenting an intriguing option. While the technique is still highly experimental, evidence is rising that women who receive their own transplanted ovarian tissue can start ovulating again and get pregnant.
“This has the potential to be one of the largest breakthroughs for reproductive choice in women,” says Pamela Madsen, executive director of the American Fertility Association, a patient-advocacy organization. “For women to be able to bank their tissue and then come back when they are ready in their lives to have their children is tremendous.”
So far, most of the hundreds of women who have frozen their ovarian tissue did so for a medical reason. Typically they were about to get chemotherapy treatment for cancer that would leave them infertile. A thornier question is whether the procedure is justified for healthy women who want to wait for pregnancy so they can advance their careers or find the right man.
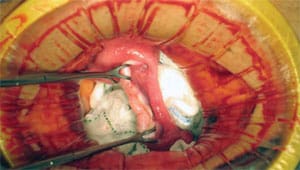
David Battaglia, a fertility specialist at Oregon Health & Science University in Portland, notes that the technique’s safety and efficacy are unproven, because only a handful of births have taken place using it. “The ethical concerns are doing a surgery on a perfectly healthy woman,” says Dr. Battaglia, who doesn’t currently recommend it to patients for nonmedical reasons. The surgery entails making a small incision below the navel to remove all or part of the ovary, and women may experience significant discomfort for several days.
Sherman Silber, Ms. Johnson’s surgeon in St. Louis, is among the pioneers of the technique and believes it should be available broadly. “Patients have to be informed and know what their options are, but if they make an informed decision who would deny them the right to preserve their fertility?” he asks.
Demand is rising, doctors say, although the procedure costs several thousand dollars. Laurel Stadtmauer of the Jones Institute for Reproductive Medicine in Norfolk, Va., says her clinic has been getting “more and more” calls from career-minded women asking about ovarian-tissue freezing and similar ways to prolong fertility. Dr. Stadtmauer doesn’t recommend tissue freezing for lifestyle reasons, though she says she’s open to offering it if patients want it.
Ethical Quandary
Because ovarian-tissue transplantation may kick-start ovulation even in a woman who has already gone through menopause, the technique raises a further ethical quandary: What should be the age limit for pregnancy? Before doing the procedure in older women, “we have first to think about what we are going to accept and not accept,” says Isabelle Demeestere, whose group works on the technique at the Free University of Brussels.
A woman is born with roughly a million immature eggs in her ovaries. Each month, beginning with puberty, one will mature for ovulation. But hundreds of the immature eggs will also die every month. A woman in her teens has about 400,000 immature eggs in her ovaries. That dwindles to 25,000 by age 37, and about 1,000 by 51. Cells surrounding the eggs contain the hormones that drive ovulation. Once they run out, the hormones do too, and a woman enters menopause.
As years go by, the quality of the remaining eggs also declines, making it harder to get pregnant with a healthy baby. “The most heartbreaking thing is speaking to 38, 39 and 40-year-old women who find out that their ovarian reserve is low or nonexistent and that the only way they could have a child is through egg donation,” says Ms. Madsen of the patient-advocacy organization.
The point of ovarian-tissue freezing is to bypass this otherwise inescapable drop in the quantity and quality of eggs. Doctors remove the millimeter-thick outer shell of the ovary, which contains the immature eggs. They divide the shell into strips and place the strips in a kind of antifreeze, and then chill it to about minus 180 degrees Celsius. In theory, the immature eggs should retain their youth indefinitely. When reimplanted in a woman, the strips can recruit their own blood supply and start producing mature eggs.
In the 1990s, Scottish researchers tested the procedure in sheep. In one study, they first removed the ovaries from eight animals, and froze several slices of tissue from the ovaries. A month later, the tissue was thawed and four ovarian strips were transplanted back into each sheep. During the following breeding season, they all had regular ovulation cycles, and one became pregnant with triplets. Scientists achieved similar success in mice and rabbits.
The first significant demonstration in humans came in 1999. A 29-year-old woman had had her ovaries removed due to medical conditions years earlier, and strips of tissue from one of them were frozen and banked. New York fertility researcher Kutluk Oktay transplanted that tissue back into the woman and reported that it began to function again.
Since then, doctors have reported two babies born with the technique, one in Belgium and one in Israel. Some controversy surrounds the Belgian case because it’s possible the fertilized egg came from an existing ovary, not the transplanted ovarian tissue.
A third pregnancy, yet to be reported in a medical journal, is ongoing, with a due date this summer. It involves a woman who had banked ovarian tissue in 1999, before chemotherapy for Hodgkin’s disease. The woman has had tissue reimplanted twice: first in 2004, after which she began to ovulate and got pregnant but miscarried, and last year, which resulted in another pregnancy. “This procedure proves that it’s possible to restore fertility,” says Dr. Demeestere in Brussels, whose team did the transplant.
The success rate of the technique remains uncertain. While hundreds of women have banked ovarian tissue, most haven’t decided to thaw it yet, leaving little way to judge the percentages. “I think we need an awful lot more data out there to see whether it works or not,” says Dr. Battaglia of Oregon Health & Science University.
Dr. Silber, who treated Ms. Johnson and directs the Infertility Center of St. Louis at St. Luke’s Hospital, believes the procedure has a good chance of working if women freeze tissue in their 20s or early 30s.
One basis for that judgment is his success in a related type of surgery: ovarian tissue transplants between identical twins. In this surgery, a woman who has become infertile receives tissue from her fertile twin. The difference with Ms. Johnson’s procedure is that the tissue needn’t be frozen because it is quickly transferred from one twin to the other.
In 2005, Dr. Silber and his colleagues reported in the New England Journal of Medicine a successful pregnancy and birth in a twin. Stephanie Yarber had undergone menopause prematurely in her teens. She received ovarian tissue from her identical twin sister at age 24. Not long thereafter she started to ovulate — the eggs came from her sister’s tissue — and she got pregnant during her second menstrual cycle, giving birth to a healthy baby girl.
Since then, Dr. Silber has worked with other sets of twins. In a report last month in the New England Journal of Medicine, he wrote that in six additional cases women began to get their periods and ovulate within months of receiving ovarian tissue from their twins. Three of those six conceived, and Ms. Yarber got pregnant again.
Good Odds
A study by researchers at the University of Valencia in Spain suggests the odds of the transplanted tissue working are fairly good. The study, published last month in the journal Human Reproduction, involved 12 female volunteers who couldn’t have children because they needed their uterus removed for medical reasons. As part of the study, the volunteers had both ovaries removed. Doctors took the shell of the left ovary containing immature eggs and transplanted it to where the right ovary had been. After two years, 11 of the 12 women were ovulating regularly, although six of them needed several months to get back to normal.
Dr. Silber’s clinic is a rare example of a major U.S. clinic offering ovarian tissue freezing to healthy women who plan to get pregnant later in life. Doctors in the field say they don’t know of anyone else doing that. “What is the difference between losing your fertility from aging of your ovary or from cancer treatment? Either way you are losing your fertility, and freezing either your eggs or your ovarian tissue can preserve that fertility,” says Dr. Silber.
A perpetually busy 65-year-old, he works some 80 to 100 hours a week and travels constantly. Colleagues describe him as exceptionally skilled in stitching together some of the body’s smallest and thinnest structures. In the 1970s, Dr. Silber pioneered vasectomy reversals, designing a technique to unblock a man’s sperm duct, which is about a hundredth of an inch thick.
Earlier this year he transplanted a whole ovary from one woman to her infertile sister, a challenging procedure because the vessels used to reattach the organ are extremely small. Theoretically, a whole-ovary transplant, by ensuring an immediate blood supply, could be superior to an ovarian-tissue transplant, but the surgery is so difficult that it’s unclear if it can ever become widely used.
Some think that Dr. Silber is going too far by offering ovarian-tissue freezing to the general public. “How can the patient be informed if you really don’t know the true success rates?” asks Dr. Oktay, the surgeon in New York. He fears patients may have exaggerated expectations about their chances of getting pregnant in later years.
Dr. Oktay heads a group at the American Society for Reproductive Medicine that is updating guidelines for fertility preservation. Current guidelines at the society, the main organization for fertility specialists, recommend against ovarian-tissue freezing for lifestyle reasons.
Ms. Johnson, a self-described biology buff, considers herself a well-informed patient. She says Dr. Silber explained to her the pros and cons of the procedure.
One option that Ms. Johnson and others in her position typically consider is freezing mature eggs. This is different from freezing ovarian tissue, which contains thousands of immature eggs but none that are ready to be fertilized by sperm. The end result, however, is similar: Both procedures may enable women to take advantage of the fertility of their younger years when they’re older.
To boost their production of mature eggs, women undergo rounds of hormone shots. Doctors then retrieve the eggs with a minimally invasive procedure and freeze them. When the woman is ready to get pregnant, sperm are introduced to the eggs through in-vitro fertilization, and the resulting embryo is implanted in the womb.
Experience with egg freezing is growing, and about 200 babies world-wide have been born with the technique, says Dr. Oktay. Initial pregnancy rates were low, but Dr. Oktay and others say the success rate is now up to 40% thanks to improved freezing techniques.
Dreading her body’s reaction to the hormone treatments, Ms. Johnson decided against egg freezing. Cost was also an issue. She paid about $5,000 for the ovarian banking procedure, while egg-freezing treatments can cost $15,000 or more. Still, her recovery from the surgery to remove her ovarian tissue wasn’t easy, and she says it took her a few weeks to feel like she was back to normal.
More Challenges
Other challenges remain. As many as half of the immature eggs in transplanted ovarian tissue die after it is reattached, limiting the life of the transplant. Freezing and thawing the tissue may damage the eggs because they are sensitive to ice formation.
The biggest threat to egg survival is lack of blood flow to the tissue in the first day or two after surgery, before new blood vessels grow into the transplant. Ms. Yarber began to run out of eggs three years after her first transplant from her twin sister. Last month, she got a new transplant of ovarian tissue from her sister, Dr. Silber says.
Centers around the world are trying to solve those problems. One new freezing technique called vitrification is yielding encouraging results in blocking ice crystals from forming on eggs and destroying them.
Perhaps the biggest obstacle to the technique’s widespread adoption is a social one. “We’ve had people in their late 30s and early 40s say they want to store their tissue, but by then it’s too late,” says Roger Gosden, laboratory director at Cornell University’s Center for Reproductive Medicine and Infertility. “The technique needs to be taken up at a younger age, and whether young women will be sufficiently concerned [about fertility] in their early 20s, I don’t know.”
See also:
News Coverage of an Whole Ovary Transplant Between Non-identical Sisters
Preserving Your Fertility
Ovarian Tissue Freezing
In Vitro Fertilization (IVF)
ICSI
Vitrification [see technical video]



